Methods and compositions for safe and effective thrombolysis
A technology of composition and use, applied in the field of safe and effective thrombolysis, which can solve the problems of delayed treatment and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
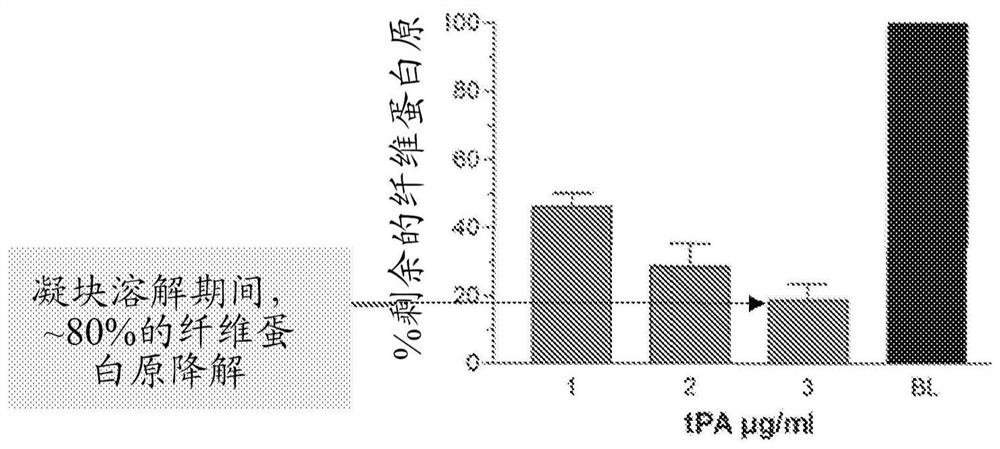
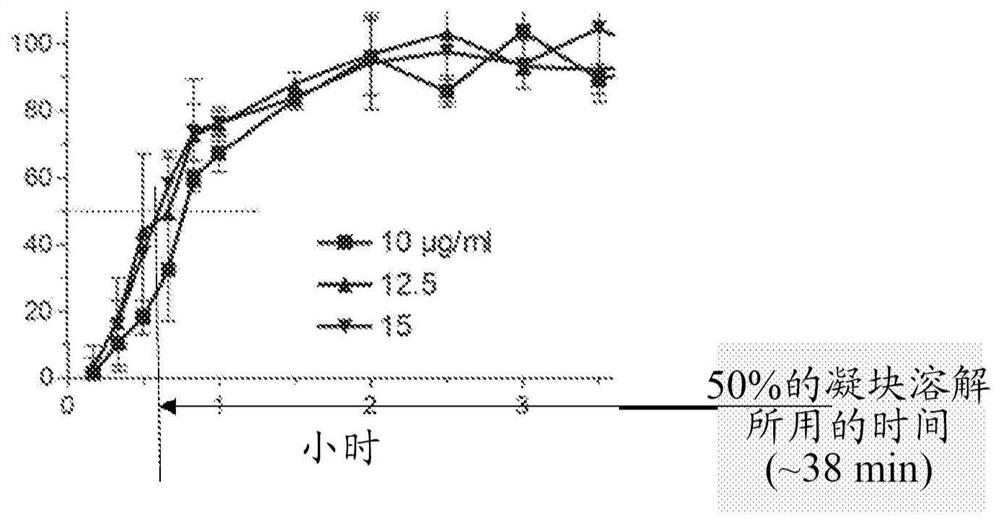
[0095] Example 1. Maximum clot lysis rate of in vitro thrombolysis induced by tPA / M5
[0096] The fibrinolytic and fibrinolytic effects of specific combinations of tPA and M5 were studied in the context of human plasma in vitro.
[0097] Material
[0098]mproUK(M5) comprising the replacement of lysine at amino acid position 300 of prourokinase with histidine (Lys300→His) was prepared from E. coli by the company PxTherapeutics (Grenoble, France). Tissue plasminogen activator (tPA) was obtained from Genentech (South San Francisco, CA). Human fibrinogen (Kabi, grade L) was obtained from Chromogenix, Milan, Italy. Aprotinin and fluorescein isothiocyanate were obtained from Sigma Chemicals (St. Louis, MO). Thrombin (ThromboMax, 100 NIH U / ml) was obtained from Sigma (St Louis, MO). Clinical grade C1-inhibitors were obtained from CSL Behring, Marburg, Germany. Pooled human expired blood bank plasma from four donors was used in the experiments.
[0099] Note that various anima...
Embodiment 2
[0126] Example 2. In vivo thrombolysis by combination of tPA and M5
[0127] Anesthetize a male mutt weighing 10-15 kg with sodium pentobarbital and maintain breathing chamber air. Blood clots were formed from 1 ml of native whole canine blood (as described in US Patent No. 7,074,401), to which radiolabeled fibrinogen (1.9 μCi, 0.75 mCi / mg protein) and thrombin (10 units) were added. After 20 min, the clot was washed 3 times with brine, and then cut into small pieces (about 1mm 3 ) and injected into the femoral vein through a 16G needle. After 15 min, a blood sample was obtained from a cannula in the contralateral femoral vein for measurement of baseline radioactivity.
[0128] Dogs were divided into four groups and injected with: (1) saline, (2) 2-5 mg tPA bolus, (3) intravenous infusion of M5 (20 μg / kg / min) for 60 min, or (4) 2-5 mg tPA bolus dose, followed by intravenous infusion of M5 (20 μg / kg / min) for 60 minutes. Blood samples were obtained at intervals during the in...
Embodiment 3
[0129] Example 3. Characterization of C1-inhibitors and M5 in a rat model of intracerebral hemorrhage (ICH)
[0130] The aim of this study was to investigate the effect of M5 on the volume of intracerebral hemorrhage (ICH).
[0131] Twenty adult male Sprague-Dawley rats were used for the study. Rats were randomly selected for the day of surgery. Rats were given a unique identification number by tail tag. Animals received cefazolin sodium intraperitoneally (40 mg / kg; Hospira 101C049) and buprenorphine subcutaneously (1 mg / kg; Reckitt Benckiser, 219202) immediately before the start of surgery. While rats under isoflurane anesthesia (1.5%-2%) breathed spontaneously inside a nitrous oxide / oxygen mixture (2:1), a small burr hole was drilled and 10 μL of 30G was microinjected into A needle (Hamilton, 700 series) was slowly lowered into the right striatum at the following coordinates from bregma: 0.0 mm anteriorly, 3 mm laterally and 6 mm deep. Over a period of 3 min, 3 μL of sal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com