Autophagy blocking system based on nano materials, preparation method thereof and application in arsenical-drug solid-tumor treatment with same
A nanomaterial and autophagy technology, which is applied in the application field of arsenic drugs in the treatment of solid tumors, can solve the problems of unsatisfactory treatment effects of arsenic drugs, and achieve good medical application prospects, good therapeutic effects, and improved therapeutic effects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
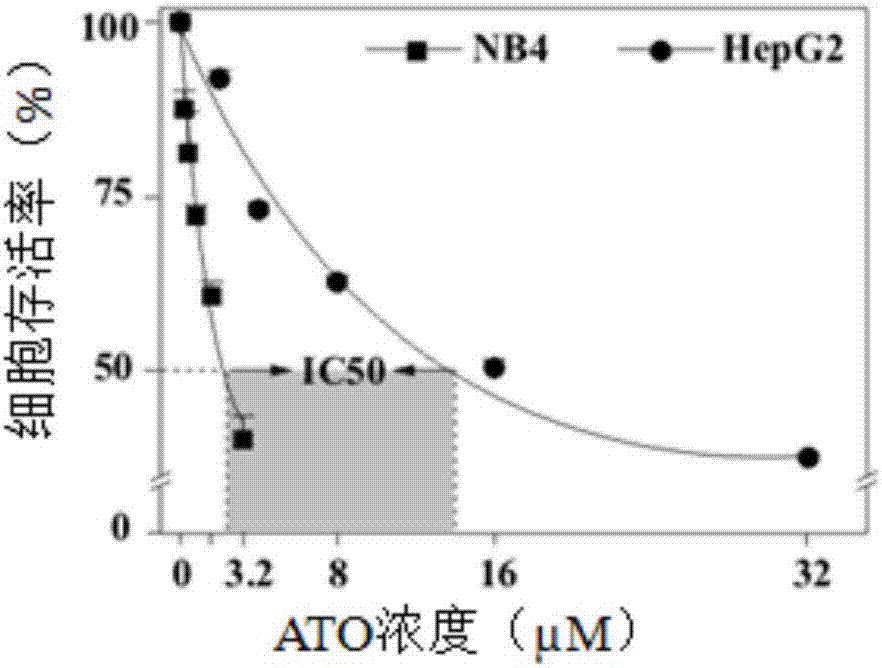
[0046] Example 1: Comparison of the efficacy of ATO on acute promyelocytic leukemia cells NB4 and human liver cancer cells HepG2
[0047] ATO was purchased from Sigma (Cat. No.: 202673), dissolved in 0.1M NaOH solution, adjusted to pH 8 with 1M HCL, filtered and sterilized.
[0048] Human liver cancer cells HepG2 were purchased from Shanghai Cell Bank of Chinese Academy of Sciences and cultured in 1640 medium (Gibco) containing 10% fetal bovine serum. 37°C, 5% CO 2 , Saturated humidity cultivation. 7×10 4 / Well density was seeded in a 24-well plate and adhered overnight. Add ATO at concentrations of 2, 4, 8, 16, 32μM respectively, with cells without any treatment as a control, three replicate wells in each group, after 48h incubation, staining with MTT (Sigma) for 4h, adding 10% acidic SDS to dissolve it Formazan crystals, the ultraviolet absorption value of each hole was measured at OD 570nm, and the cell survival rate was expressed as the percentage of OD (treatment group) / OD (...
Embodiment 2
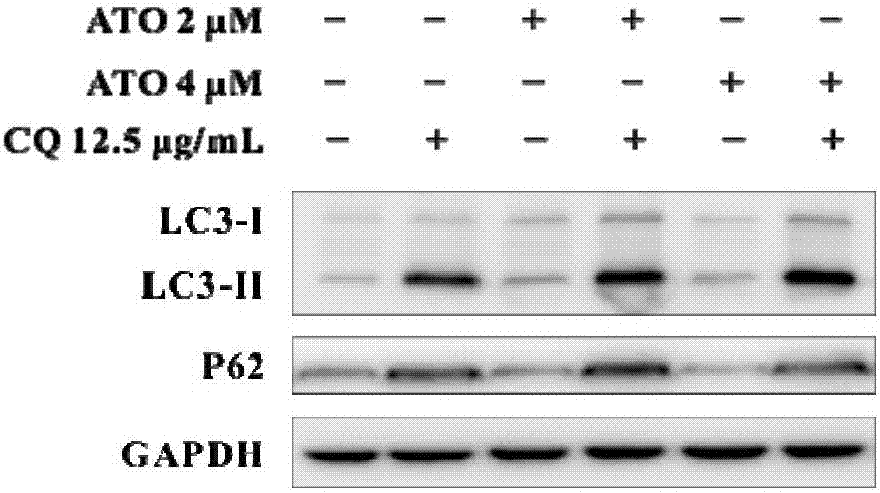
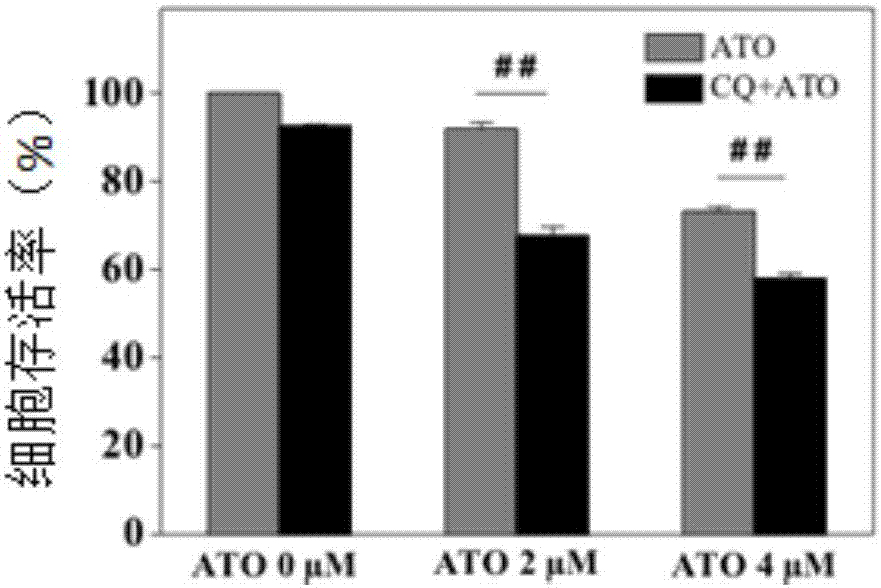
[0051] Example 2: Evaluation of the efficacy of ATO combined with autophagy blocking drug CQ on tumor cells.
[0052] CQ was purchased from Sigma (Cat. No.: C6628) and dissolved in sterile water.
[0053] In western blotting experiments, HepG2 cells were 7×10 4 The density of / well was inoculated in a 24-well plate and adhered overnight. Set up the following experimental groups, each with three replicates: CQ (12.5μg / mL), ATO (2μM and 4μM each), CQ+ATO mixture (12.5μg / mL CQ+2μM ATO and 12.5μg / mL CQ+ 4μM ATO each group), with the cells without any treatment as a control, incubate for 48h. Wash the cells twice with PBS, lyse the cells with 1×SDS loading buffer, denature at 95°C, transfer to PVDF membrane after polyacrylamide gel electrophoresis. The PVDF membrane was blocked with 6% skimmed milk powder dissolved in PBST (0.1% Tween 20) for 1 hour, washed with PBST 3 times, and a 1:1000 primary antibody was added. The primary antibodies involved are as follows: anti-LC3 (Novus), P6...
Embodiment 3
[0056] Example 3: Nano-diamond's autophagy regulation effect on cells.
[0057] Nanodiamonds (NDs, Gansu Jinshi Nanomaterials Co., Ltd.), the purity of the nanodiamonds> 99%, the single particle diameter is about 2-10nm, forming a cluster structure of about 250nm in the solution. Irradiate with ultraviolet for 30 minutes and disperse in sterile water.
[0058] In western blotting experiments, HepG2 cells were 7×10 4 The density per well was seeded in a 24-well plate and adhered overnight. 50μg / mL NDs and 12.5μg / mL CQ were added respectively, and the cells without any treatment were used as controls. Three replicate wells in each group were incubated for 48h. The methods of cell lysis, protein electrophoresis, and membrane transfer are the same as in the second embodiment. The primary antibodies involved in this example are as follows: anti-LC3 (Novus), P62 (Abcam) and GAPDH (Abcam), mTOR (cell signaling technology), P-mTOR (cell signaling technology), p70S6K (cell signaling techn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com