Soluble recombinant protein and expression and purification methods and application thereof
A recombinant protein and expression method technology, applied in the field of recombinant proteins, can solve the problems of unsuitable large-scale production, complex preparation process, poor immunogenicity, etc., and achieve the effects of being suitable for large-scale industrial production, improving the purity, and being easy to operate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: Construction of the soluble recombinant protein expression vector of the present invention
[0036] 1.1 Acquisition of Cap protein gene
[0037] Based on the sequences of PCV2 epidemic strains in the NCBI database, after comparison, the Cap protein amino acid sequence of a epidemic strain was selected, which was identical or highly similar to the corresponding sequences of other epidemic strains. As shown in SEQ ID NO:1. Under the premise of ensuring that the amino acid of the Cap protein of this strain remains unchanged, the common codons of Escherichia coli are used to translate the amino acid sequence into a nucleotide sequence, and the nucleotide sequence is shown in SEQ ID NO: 2, making it suitable for use in Escherichia coli Expression, and then entrust Jinweizhi Company to carry out gene synthesis. During gene synthesis, restriction sites are added at both ends. The nucleotide sequence after adding restriction sites is as SEQ ID NO: 3, and the synt...
Embodiment 2
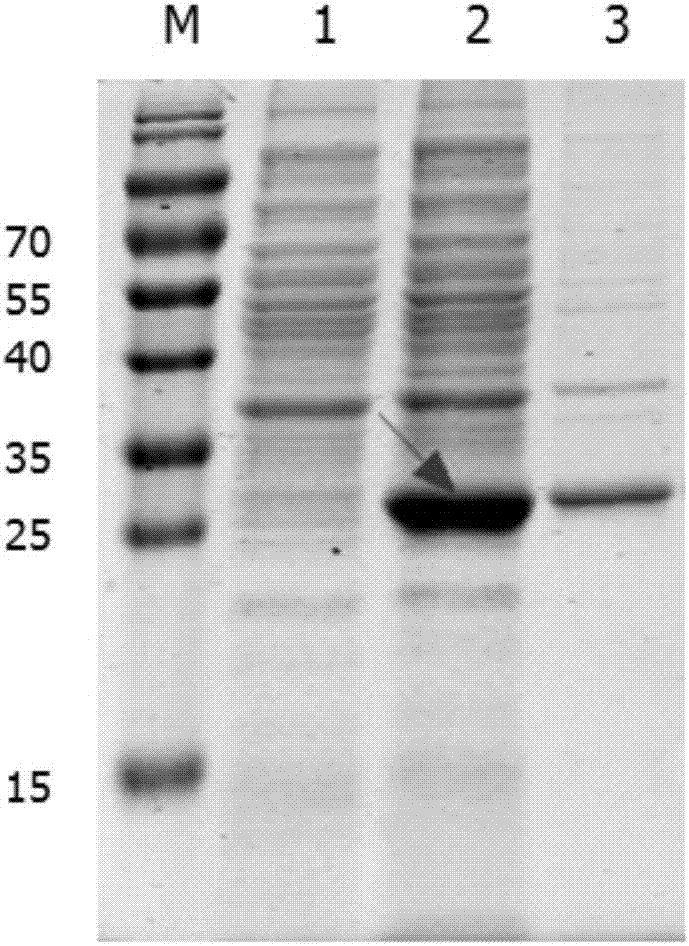
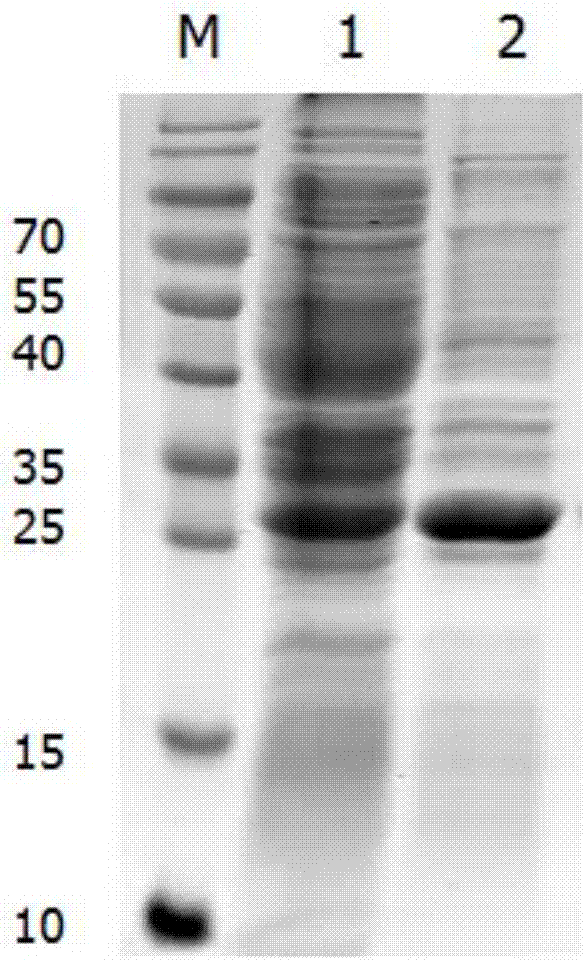
[0042] Embodiment 2: Expression and purification of the soluble recombinant protein of the present invention
[0043] 2.1 Mass expression of soluble recombinant protein
[0044] (1) Take out the Escherichia coli strain carrying the recombinant plasmid pET-28a-Cap-gene from -20°C, insert it into kanamycin sulfate-resistant LB liquid medium at a ratio of 1:1000, and cultivate overnight on a shaker at 220rpm at 37°C (12-14h).
[0045] (2) Calibrate the PH electrode of the 5L fermentation tank, prepare 2.5L medium and put it into the tank, sterilize at 121°C for 20 minutes, connect the automatic control system after cooling, correct the dissolved oxygen, adjust the ventilation and the initial speed.
[0046] (3) 10% seed liquid is connected to the fermenter, the temperature is 37°C, the stirring speed and ventilation are manually adjusted, and the dissolved oxygen is controlled between 30% and 60%.
[0047] ⑷ When the dissolved oxygen starts to rise rapidly, add the feeding medi...
Embodiment 3
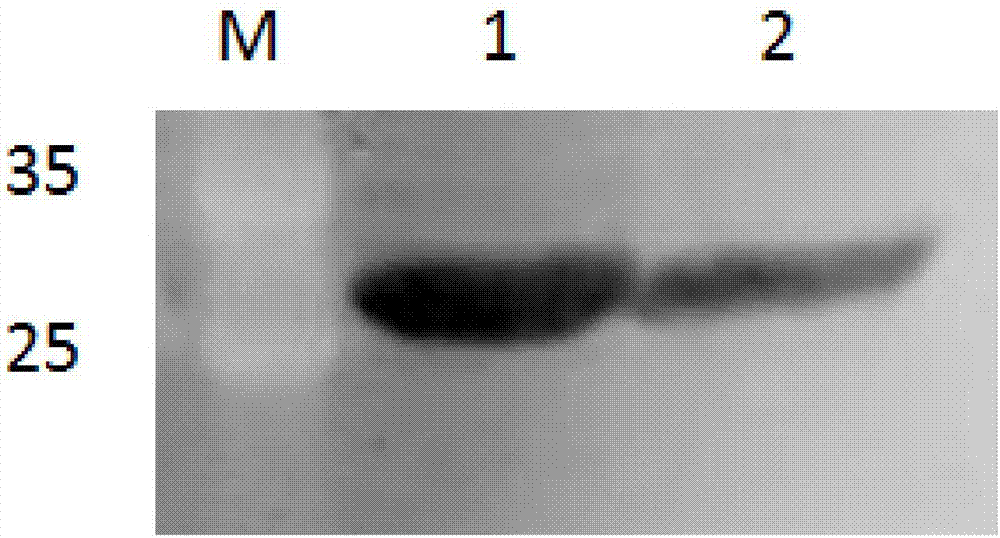
[0061] Embodiment 3: the preparation of PCV2 virus-like particle vaccine of the present invention
[0062] First, use the BCA kit to measure the above-mentioned purified soluble recombinant protein, adjust the concentration of the soluble recombinant protein to 1mg / ml, and then mix the protein with 201 adjuvant provided by Sepic company in France according to the volume ratio of 1:1, and then carry out Phacoemulsification. Store at 4°C after testing the viscosity and stability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com