High-strength chemical-physical dual-network hydrogel with self-recovery capability, and preparation method and application of hydrogel
A hydrogel and self-recovery technology, which is applied in the fields of medical preparations of non-active ingredients, medical science, and pharmaceutical formulations, can solve the problems of low gel fracture energy and decreased mechanical properties, and achieve simple preparation process and recovery of mechanical properties. Performance, Effect of Good Fatigue Resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
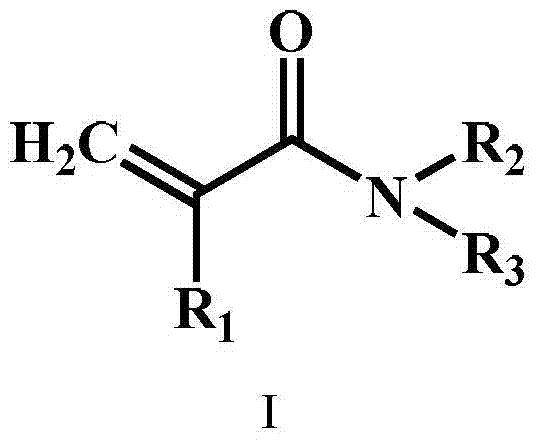
[0057] (1) Weigh 50 mg of chitosan, 15 mg of the first cross-linking agent N,N'-methylenebisacrylamide, and 2-hydroxy-4-(2-hydroxyethoxy)-2-methylbenzene as the initiator Acetone 5mg, hydrophilic monomer acrylamide 800mg, then add 10mL of water, evenly mix and dissolve to obtain a mixed solution; pour this mixed solution into a glass mold, and irradiate it under a 100-watt UV lamp for 30 minutes to obtain the first chemical network hydrogels;
[0058] (2) Then, 20 g of potassium chloride was dissolved in 100 mL of water to prepare a second crosslinking agent solution. After soaking the first chemical network hydrogel in KCl solution for 2 hours, the gel changed from transparent to opaque chemical-physical dual network hydrogel.
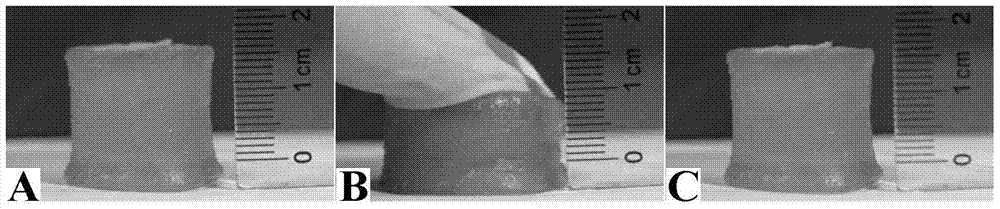
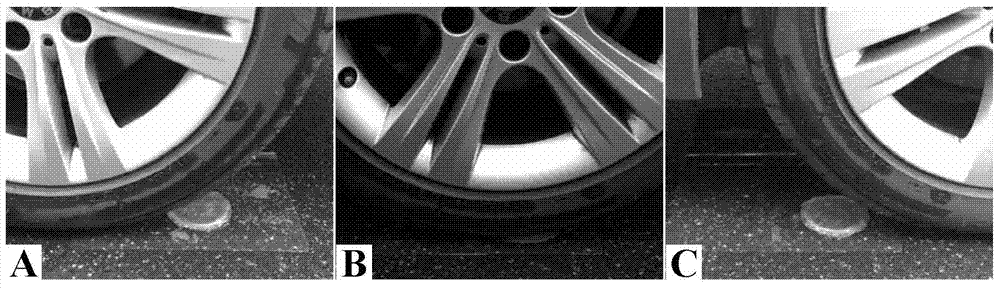
[0059] The obtained chemical-physical double network hydrogel was tested, the compressive strength was 150MPa, the compressive deformation rate was over 99%, the tensile strength was 5.05MPa, and the elongation at break was 886%. The gel can be rest...
Embodiment 2
[0062] (1) Weigh 75 mg of chitosan, 10 mg of the first cross-linking agent N,N'-methylenebisacrylamide, 5 mg of ammonium persulfate initiator, 1000 mg of hydrophilic monomer acrylic acid, and then add 10 mL of water, uniformly Mix and dissolve to obtain a mixed solution; pour the mixed solution into a glass mold, and heat it in a water bath at 60°C for 5 hours to obtain a first chemical network hydrogel;
[0063] (2) Then, 20 g of magnesium nitrate was dissolved in 100 mL of water to prepare a second crosslinking agent solution. After soaking the first chemical network hydrogel in magnesium nitrate solution for 4 hours, the gel changed from transparent to opaque chemical-physical dual network hydrogel.
[0064] The obtained chemical-physical double network hydrogel was tested, and the compressive strength was 180MPa, the compressive deformation rate was over 99%, the tensile strength was 6.05MPa, and the elongation at break was 736%. The gel can be restored to its original te...
Embodiment 3
[0066] (1) Weigh 40 mg of chitin, 15 mg of the first cross-linking agent N,N'-methylenebisacrylamide, 3.5 mg of the initiator α-ketoglutaric acid, and the hydrophilic monomer methacrylic acid N,N- 1200 mg of dimethylaminoethyl ester, then 10 mL of water was added, and the mixture was uniformly mixed and dissolved to obtain a mixed solution; the mixed solution was poured into a glass mold and irradiated under a 150-watt UV lamp for 40 minutes to obtain the first chemical network hydrogel ;
[0067] (2) Then, 1.5 g of sodium hydroxide was dissolved in 100 mL of water to prepare a second crosslinking agent solution. The first chemical network hydrogel was soaked in sodium hydroxide solution for 5 hours, and the gel changed from transparent to translucent chemical-physical dual network hydrogel.
[0068] The obtained chemical-physical double network hydrogel was tested, and the compressive strength was 160 MPa, the compressive deformation rate was over 99%, the tensile strength was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| compressive strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com