Method for preparation of methyl cyclohexanediamine by selective hydrogenation of toluenediamine and catalyst
A technology for producing methylcyclohexanediamine and toluenediamine, which is applied in the field of selective hydrogenation of toluenediamine to produce methylcyclohexanediamine, which can solve the problems of high catalyst preparation cost, many steps in the preparation process, and easy deactivation of the catalyst. problem, to achieve the effect of simple preparation method, high selectivity, and improved activity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
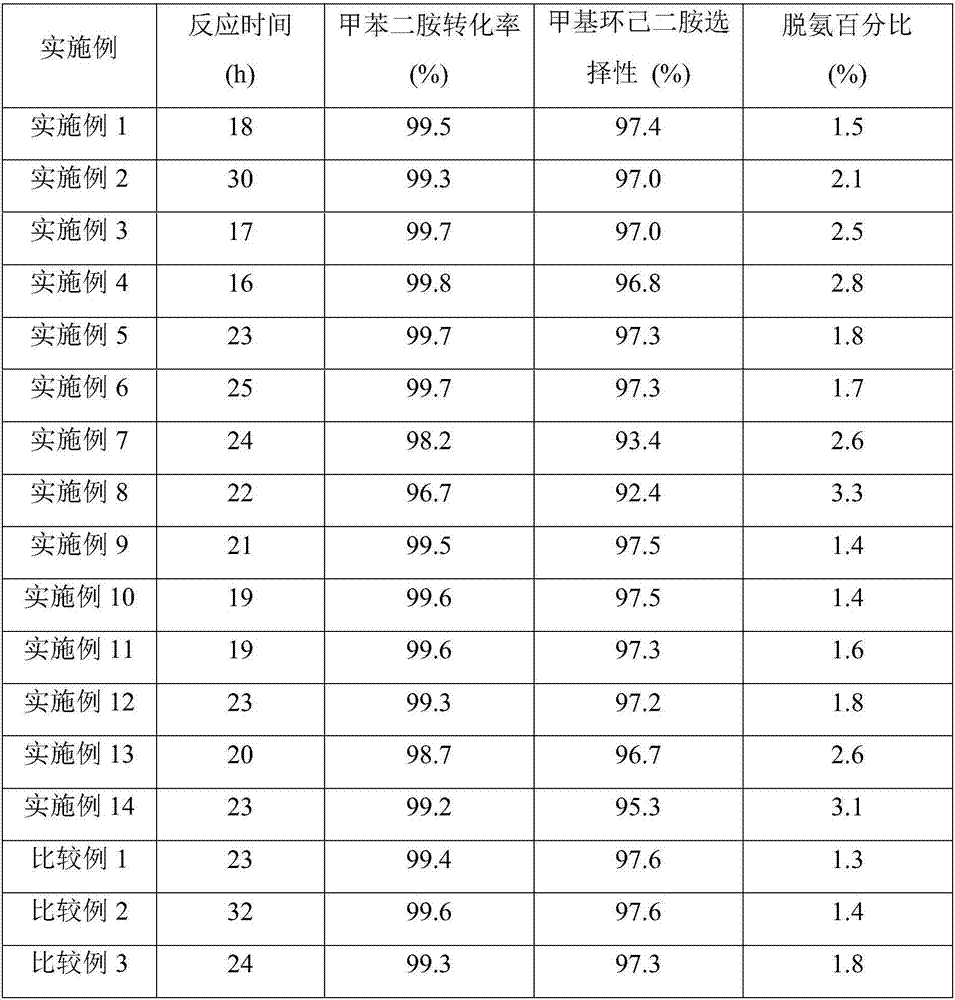
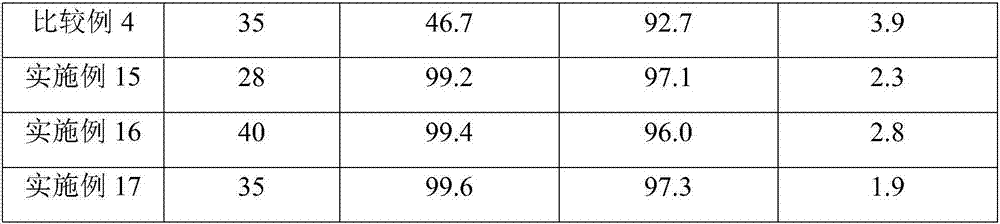
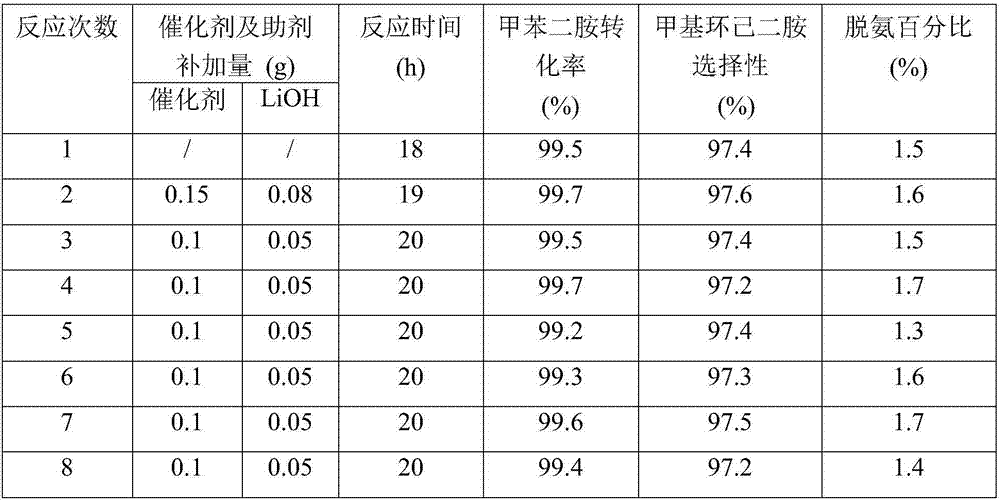
Embodiment 1
[0052] Catalyst preparation: Weigh 10g of alumina carrier, dissolve it in an aqueous solution containing a certain amount of ruthenium chloride and cerium nitrate, add carrier mass fraction 3% polyethylene glycol into the impregnating solution, impregnate at room temperature for 12h, and then stir at 100°C , Evaporate to dryness. The obtained sample was dried at 120°C for 6 hours, calcined at 450°C for 4 hours, and reduced with hydrogen at 200°C for 3 hours to obtain a supported catalyst 1.0%Ce-5%Ru / Al 2 o 3 .
[0053] Hydrogenation reaction: carried out in a 300mL autoclave: 30.0g industrial grade TDA, 90.0g tetrahydrofuran, 1.5g catalyst 1.0%Ce-5%Ru / Al 2 o 3 , add 0.3g of lithium hydroxide to a 300mL reactor, replace the air in the reactor with nitrogen for 3 times, replace the air in the reactor with hydrogen for 5 times, control the reaction temperature at 180°C, the reaction pressure at 8.0MPa, and the stirring speed at 500 rpm to The hydrogen in the kettle is basical...
Embodiment 2
[0055] Catalyst preparation: Weigh 10g of alumina carrier, dissolve it in an aqueous solution containing a certain amount of ruthenium chloride and cerium nitrate, add carrier mass fraction 3% polyethylene glycol into the impregnating solution, impregnate at room temperature for 12h, and then stir at 100°C , Evaporate to dryness. The obtained sample was dried at 120°C for 6h, calcined at 450°C for 4h, and reduced with hydrogen at 200°C for 3h to obtain a supported catalyst 1.0%Ce-2%Ru / Al 2 o 3 .
[0056] Hydrogenation reaction: except using the catalyst prepared in this embodiment, all the others are the same as in embodiment 1.
Embodiment 3
[0058] Weigh 10g of alumina carrier, dissolve it in an aqueous solution containing a certain amount of ruthenium chloride and cerium nitrate, add carrier mass fraction 3% polyethylene glycol into the impregnating solution, impregnate at room temperature for 12h, then stir and evaporate to dryness at 100°C moisture. The obtained sample was dried at 120°C for 6h, calcined at 450°C for 4h, and reduced with hydrogen at 200°C for 3h to obtain a supported catalyst 1.0%Ce-10%Ru / Al 2 o 3 .
[0059] Hydrogenation reaction: except using the catalyst prepared in this embodiment, all the others are the same as in embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com