2-(9-methylene anthracene) thiosemicarbazide and TAT modified gold nanoparticle drug delivery system and application
A technology of methylene anthracene and thiosemicarbazide, which is applied in the field of biomedicine, can solve problems such as the application limitation of chemotherapeutic drugs, and achieve the effects of good biocompatibility, low cytotoxicity and enhanced Raman signal.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
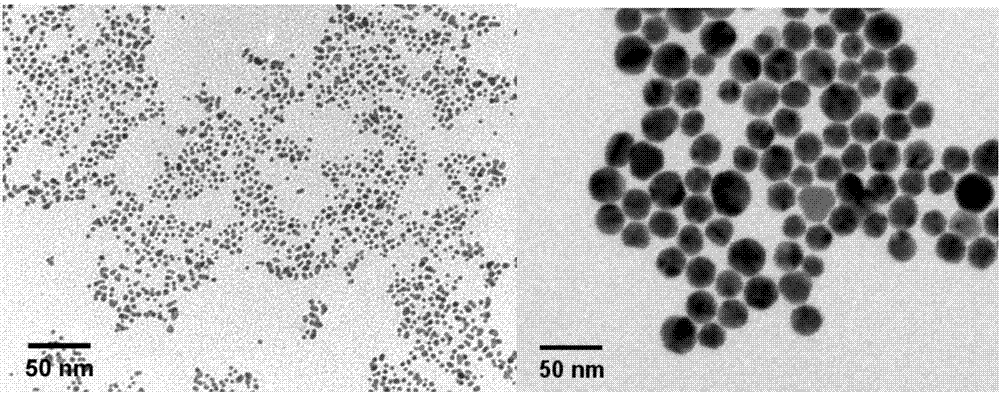
[0033] Example 1 ANS-TAT-AuNPs 3.8nm and its preparation method
[0034] The particle size synthesized in this example is a gold nanometer drug-carrying system of 3.8nm, and the preparation method comprises the following steps:
[0035] (1) Preparation of gold nanoparticles coated with citrate ions by sodium borohydride reduction method: in 0.25mM HAuCl 4 and 0.25mM sodium citrate mixed aqueous solution 200mL, add ice-bathed 0.1M NaBH 4 Aqueous solution 6mL, stirred at room temperature for 2h in the dark;
[0036] (2) TAT modification: Add 1mL of 1mM polypeptide TAT aqueous solution to 197mL of the solution prepared in step (1), and stir at room temperature for 1min;
[0037] (3) Coating of 2-(9-methylene anthracene) thiosemicarbazide: 15 mL of DMSO solution of 0.2 mg / mL 2-(9-methylene anthracene) thiosemicarbazide was added to the solution prepared in step (2), Stir at room temperature in the dark for 2 hours; let stand, wash several times with water, centrifuge, and quan...
Embodiment 2
[0039] Example 2 ANS-TAT-AuNPs 22.1n and its preparation method
[0040] The particle size synthesized in this example is a gold nano drug-carrying system of 22.1nm. The preparation method comprises the following steps:
[0041] (1) Preparation of gold nanoparticles coated with citrate ions by sodium citrate reduction method: in 0.23mM HAuCl 4 Add 10mL of 1% sodium citrate aqueous solution to 192mL aqueous solution, stir, reflux for 2h, and set aside;
[0042] (2) TAT modification: add 43.95 μL of 0.1 mM polypeptide TAT aqueous solution to the solution prepared in step (1), and stir at room temperature for 1 min;
[0043] (3) Coating of 2-(9-methyleneanthracene)thiosemicarbazide: 10 mL of 0.05 mg / mL 2-(9-methyleneanthracene) thiosemicarbazide in DMSO and 0.2 mg / mL 2-(9-methanthracene) Add 5 mL of DMSO solution of methyl anthracene) thiosemicarbazide to the solution prepared in step (2), and stir at room temperature in the dark for 2 h; let stand, wash several times with water...
Embodiment 3
[0046] Example 3 Determination of drug resistance index of ANS-TAT-AuNPs
[0047] The drug resistance index of ANS-TAT-AuNPs with different particle sizes was measured, and the experimental method adopted the conventional MTT method.
[0048] 1. Sample preparation: Dissolve ANS-TAT-AuNPs in a mixed solution of DMSO / water and dilute with culture medium.
[0049] 2. Cell lines: MCF-7 sensitive cells and MCF-7 / ADR drug-resistant cells, the above cell lines are all frozen and passaged by our laboratory.
[0050] 3. Culture medium: RPMI 1640+10% fetal bovine serum+double antibody (penicillin, streptomycin each 100U / mL).
[0051] 4. Other materials: automatic microplate reader, model PerkinElmer EnSpire 2300Multilabel PlateReader, 96-well plate.
[0052] 5. Test method: MTT method. Add 5×10 per well in a 96-well plate 4 100 μL / mL of cell suspension, placed at 37 °C, 5% CO 2 in the incubator. After 24 hours, the culture medium was aspirated and replaced with drug-containing med...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com