Application of lobaplatin in preparing medicines for treating malignant trophoblastic tumor
A technology of trophoblasts and tumor drugs, applied in the direction of anti-tumor drugs, drug combination, drug delivery, etc., can solve the problem of no malignant trophoblastic tumor treatment effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Study on the activity of lobaplatin against trophoblast tumor cells in vitro
[0018] 1.1 Materials and reagents
[0019] 1.1.1 Drug name and source: Lobaplatin is white freeze-dried powder, Hainan Chang’an International Pharmaceutical Co., Ltd.; carboplatin is white powder, Kunming Guiyan Pharmaceutical Co., Ltd.; 5-fluorouracil, Xi’an Haixin Pharmaceutical Co., Ltd.; cisplatin It is yellow powder, Jiangsu Hansoh Pharmaceutical Co., Ltd.
[0020] Preparation method: the above drugs are formulated with corresponding concentrations in serum-free medium.
[0021] 1.1.2 Cell lines
[0022] Human trophoblastic tumor JEG-3 cells and JAR cells were purchased from the State Key Laboratory of Family Planning and Reproductive Biology, Institute of Zoology, Chinese Academy of Sciences, and cultured according to the instructions provided.
[0023] 1.1.3 Reagents and instruments
[0024] DMEM / F12 (1:1) medium, fetal bovine serum (Gibco, USA), penicillin (100u / L), str...
example 2
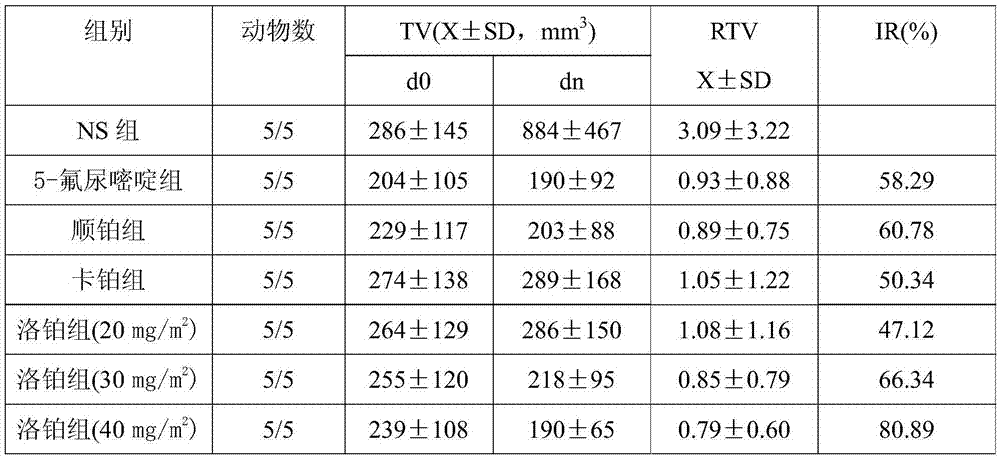
[0041] Trial Example 2: Study on In vivo Curative Effect of Lobaplatin on Trophoblastic Tumor
[0042] 2.1 Materials and reagents:
[0043]2.1.1 Drug name and source: Lobaplatin is white freeze-dried powder, Hainan Changan International Pharmaceutical Co., Ltd.; carboplatin is white powder, Kunming Guiyan Pharmaceutical Co., Ltd.; 5-fluorouracil, Xi’an Haixin Pharmaceutical Co., Ltd.; cisplatin It is yellow powder, Jiangsu Hansoh Pharmaceutical Co., Ltd.
[0044] 2.1.2 Experimental animals and cells
[0045] SCID beige female mice (3-5 weeks old, body weight 16-20g) were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.; human trophoblastic tumor cell line JAR was purchased from the American Standard Biological Collection (ATCC ), lentiviral empty vector GV208, element sequence: Ubi-MCS-EGFP, eukaryotic resistance: puromycin, fluorescent marker: EGFP, purchased from Jikai Gene, Shanghai; Polybrene was purchased from Shanghai Jikai Company.
[004...
Embodiment 3
[0066] Example 3: Clinical trials of lobaplatin in the treatment of malignant trophoblastic tumors
[0067] 3.1 Case selection: Through the clinical diagnosis of typical symptoms of malignant trophoblastic tumor, combined with HCG (human chorionic gonadotropin) detection, B-ultrasound and CT and other examinations, the patients with a definite diagnosis of malignant trophoblastic tumor were screened out 15 cases, according to UICC TNM staging, 5 cases were in stage III and 10 cases were in stage IV. All patients had a KPS score greater than 60. 15 patients were randomly divided into two groups, 8 cases in the treatment group, all female, aged 26-45 years, with an average age of 34.3 years, 2 cases in stage III, 6 cases in stage IV; 6 cases in the control group, all female, age 23-47 years old, with an average age of 35.9 years, 3 cases were in stage III, and 4 cases were in stage IV. The two groups were comparable in terms of age, gender, disease duration, and condition. Be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com