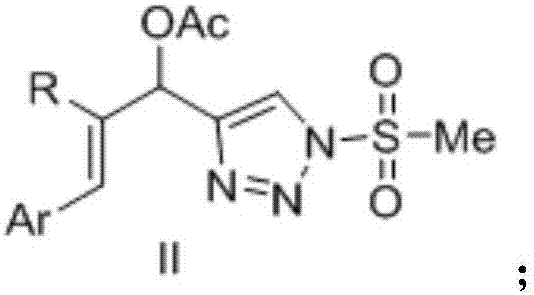
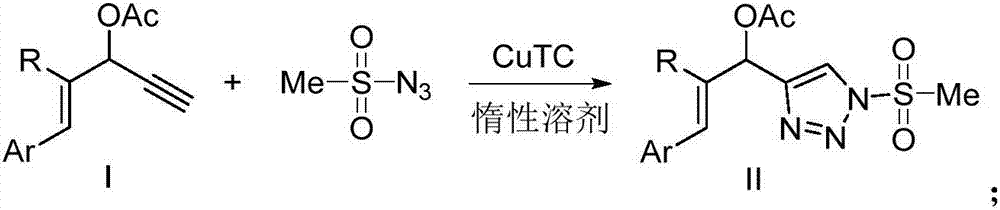
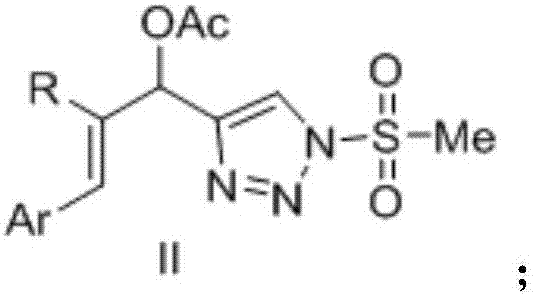
Novel 4-allyl acetate substituted N-sulfonyl 1,2,3-triazole and preparation method thereof
A kind of technology of allyl acetate and triazole, applied in the field of organic synthesis, can solve the problems such as no public report of preparation technology, and achieve the effects of high reaction efficiency and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Preparation of (E)-1-(1-methylsulfonyl-1H-1,2,3-triazole 4-alkynyl)-3-phenylallyl acetate
[0023] Under nitrogen protection, (E)-1-phenyl-1,4-enyne-3-acetate (0.4mmol, 80mg) was dissolved in toluene (2mL), and then methanesulfonyl azide (0.4 mmol, 48.4mg) and CuTC (0.02mmol, 3.8mg), the reaction mixture was stirred at room temperature, and reacted for 16h. After the reaction, saturated NH 4 Cl solution was used to quench the reaction, then extracted with organic solvent, washed with saturated NaCl solution, dried over anhydrous sodium sulfate, the combined solution was distilled under reduced pressure, and separated by column chromatography to obtain 100 mg of yellow liquid product with a yield of 78%.
[0024] 1 H-NMR (500MHz, CDCl 3 ):δ2.16(s,3H),3.56(s,3H),6.52(dd,J=15.5,1.5Hz,1H),6.61(d,J=1.5Hz,1H),6.80(d,J= 15.5Hz,1H),7.28-7.40(m,3H),7.43-7.47(m,2H),8.17(s,1H). 13 C-NMR (100MHz, CDCl 3 ): δ21.4, 43.0, 68.6, 122.3, 124.0, 127.1, 128.8, 128.9, 135.2,...
Embodiment 2
[0025] Example 2: (E)-2-methyl-1-(1-methylsulfonyl-1H-1,2,3-triazole 4-alkynyl)-3-phenylallyl acetate preparation
[0026] Under nitrogen protection, (E)-1-phenyl 2-methyl-1,4-enyne-3-acetate (0.4mmol, 86mg) was dissolved in toluene (2mL), and methanesulfonyl Azide (0.4mmol, 48.4mg) and CuTC (0.02mmol, 3.8mg), the reaction mixture was stirred at room temperature, and reacted for 16h. 4 The reaction was quenched with Cl solution, extracted with organic solvent, washed with saturated NaCl solution, dried over anhydrous sodium sulfate, the combined solution was distilled under reduced pressure, and separated by column chromatography to obtain 96 mg of yellow liquid product with a yield of 72%.
[0027] 1 H-NMR (400MHz, CDCl 3 ):δ1.89(s,3H),2.17(s,3H),3.56(s,3H),6.55(s,1H),6.67(s,1H),7.20-7.40(m,5H),8.15( s, 1H). 13 C-NMR (100MHz, CDCl 3 ): δ14.7, 21.3, 42.9, 73.2, 122.3, 127.4, 128.5, 129.3, 129.8, 133.5, 136.6, 146.4, 169.9. HRMS (ESI) Calcd for C 15 h 18 N 3 o 4 S[M+H...
Embodiment 3
[0028] Example 3: (E)-3-(3-methoxyphenyl)-1-(1-methylsulfonyl-1H-1,2,3-triazol4-alkynyl)-3-allyl Preparation of Glycolacetate
[0029] Under nitrogen protection, dissolve (E)-1-(3-methoxyphenyl)-1,4-enyne-3-acetate (0.4mmol, 92mg) in toluene (2mL), and add Methanesulfonyl azide (0.4mmol, 48.4mg) and CuTC (0.02mmol, 3.8mg), the reaction mixture was stirred at room temperature, and reacted for 16h. 4 Cl solution was used to quench the reaction, and then extracted with organic solvent, washed with saturated NaCl solution, dried over anhydrous sodium sulfate, the combined solution was distilled under reduced pressure, and separated by column chromatography to obtain 119 mg of yellow liquid product with a yield of 85%.
[0030] 1 H-NMR (400MHz, CDCl 3 ): δ2.10(s,3H),3.53(s,3H),3.79(s,3H),6.48(dd,J=15.8,1.8Hz,1H),6.60(d,J=1.8Hz,1H) ,6.72(d,J=15.8Hz,1H),6.81-6.83(m,1H),6.89-6.91(m,1H),6.98-7.01(m,1H),7.20-7.30(m,1H),8.15 (s,1H). 13 C-NMR (100MHz, CDCl 3 ): δ21.3, 42.9, 55.5, 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com