Breviscapine/chitosan composite hydrogel restraining cicatrization and preparation method thereof
A technology of compounding hydrogel and breviscapine, which is used in pharmaceutical formulation, pharmaceutical science, drug delivery and other directions to achieve the effects of improving plasticity and dispersibility, inhibiting proliferation and efficient scar formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 1. Preparation of breviscapine / chitosan composite hydrogel
[0053] (1) This example provides a breviscapine / chitosan composite hydrogel that inhibits scar formation, which is prepared from chitosan, chitosan oligosaccharides, breviscapine, polyvinyl alcohol and soluble starch, each The dosage of components is: chitosan (10KDa) 2g, chitosan oligosaccharide (3KDa) 0.3g, scutellarin 2g, polyvinyl alcohol 5g, soluble starch 1g.
[0054] (2) Preparation method
[0055] According to the above quality, the raw materials of each component are respectively selected for use, and the scutellarin / chitosan composite hydrogel is prepared. The specific method includes the following steps:
[0056] S1. Pipette 1mL of glacial acetic acid into a 1L volumetric flask, add deionized water to prepare a 1% acetic acid solution; weigh 2g of chitosan with a molecular weight of 10KDa and a deacetylation degree of 85% and dissolve it in the above acetic acid solution In, stir 30min under the r...
Embodiment 2
[0067] 1. Preparation of breviscapine / chitosan composite hydrogel
[0068] This embodiment provides a breviscapine / chitosan composite hydrogel that inhibits scar formation, which is prepared from chitosan, chitosan oligosaccharide, breviscapine, polyvinyl alcohol and soluble starch, and the dosage of each component is : Chitosan (20KDa) 2g, chitooligosaccharide (2KDa) 0.3g, scutellarin 1.5g, polyvinyl alcohol 5g, soluble starch 0.8g.
[0069] For the specific preparation method, refer to Example 1, which will not be repeated here.
[0070] 2. Test experiment of breviscapine / chitosan composite hydrogel
[0071] (1) Properties test of breviscapine / chitosan composite hydrogel
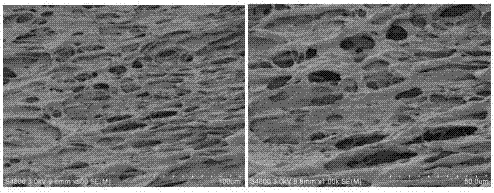
[0072] Figure 4 The scanning electron microscope image of the dried scutellarin / chitosan composite hydrogel prepared for this example shows that the hydrogel has a dense porous structure. When water is contained, these pore-like structures can store water. When the voids of the hydrogel are filled wit...
Embodiment 3
[0076] 1. Preparation of breviscapine / chitosan composite hydrogel
[0077] This embodiment provides a breviscapine / chitosan composite hydrogel that inhibits scar formation, which is prepared from chitosan, chitosan oligosaccharide, breviscapine, polyvinyl alcohol and soluble starch, and the dosage of each component is : Chitosan (10KDa) 1.5g, chitooligosaccharide (300Da) 0.1g, scutellarin 1g, polyvinyl alcohol 5g, soluble starch 0.8g.
[0078] For the specific preparation method, refer to Example 1, which will not be repeated here.
[0079] 2. Test experiment of breviscapine / chitosan composite hydrogel
[0080] (1) Properties test of breviscapine / chitosan composite hydrogel
[0081] Figure 6 The scanning electron microscope image of the dried scutellarin / chitosan composite hydrogel prepared for this example shows that the hydrogel has a dense porous structure. When water is contained, these pore-like structures can store water. When the voids of the hydrogel are filled wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com