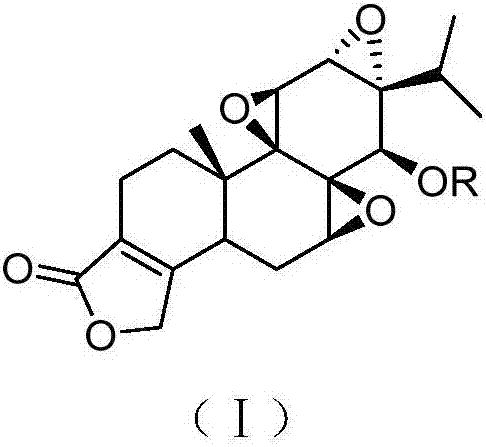
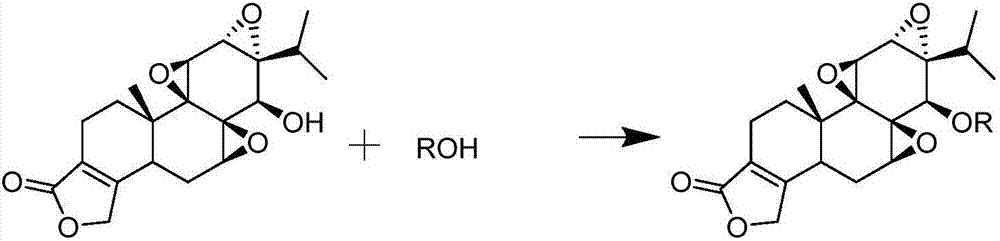
Triptolide derivative, preparation method and preparation thereof
A technology of triptolide and nano-preparation, which is applied in the field of medicine, can solve the problems of reduced drug load, poor patient compliance, and reduced encapsulation rate, and achieves the effects of improving fat solubility, enhancing fat solubility, and prolonging half-life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The preparation of embodiment 1 different triptolide fatty acid esters
[0053] 1.1.1 Preparation of triptolide stearate (TP-SA)
[0054] Dosing 3mmol of stearic acid, 3mmol of DCC, and 3mmol of DMAP in the reaction vessel, adding 20mL of anhydrous dichloromethane to dissolve, stirring for 30 minutes under ice bath conditions; dissolving 1mmol of TP in an appropriate amount of anhydrous dichloromethane and Slowly added dropwise to the reaction system, reacted under ice bath conditions for 30 minutes, continued to react overnight at room temperature, and separated and purified the reactant through a silica gel column to obtain 532.2 mg of triptolide stearate. Yield 84.9%.
[0055] 1 H NMR (DMSO- d6 ,600MHz)δ4.98(1H,s,14-CH),4.86(1H,d,J=18.88Hz,19-CH),4.77(1H,d,J=18.88Hz,19-CH),3.95( 1H,d,11-CH),3.69(1H,d,12-CH),3.56(1H,d,7-CH),2.28-2.39(2H,m,2ˊ-CH 2 ),1.73-2.00(1H,m,15-CH),1.80-1.85(2H,m,3ˊ-CH 2 ),1.57-1.59(2H,m,2-CH 2 ),1.24-1.34(28H,m,14×CH 2 ),0.92(3H,s,20-CH ...
Embodiment 2
[0115] Example 2: Preparation of different nano-preparations of triptolide fatty acid esters
[0116] The different triptolide fatty acid esters prepared in Example 1 were used to investigate the druggability of the formulations, including liposomes, polymer micelles, albumin nanoparticles, and fat emulsions.
[0117] 2.1 Preparation of different triptolide fatty acid ester liposomes
[0118] 2.1.1a Preparation of triptolide stearate (TP-SA) liposomes
[0119] Weigh 0.2g of triptolide stearate, 2g of egg yolk phospholipid (PC-98T) and 0.2g of cholesterol, add it to 5g of absolute ethanol, heat and dissolve at 60°C to obtain an organic phase; slowly inject the organic phase into an 80mL Inject water at 60°C, stir and mix evenly while injecting to obtain the crude liposome; place the crude liposome in an extruder, and extrude through extrusion membranes with pore diameters of 0.2 μm, 0.1 μm, and 0.05 μm in sequence, Obtain the liposome solution; remove the ethanol in the lipos...
Embodiment 3
[0194] Embodiment 3: the mensuration of liposome particle size and encapsulation efficiency
[0195] Different triptolide fatty acid esters and triptolide liposomes were prepared respectively by taking the drug-to-lipid ratio in Example 2.1.1b as a unified prescription, and the liposome particle size and encapsulation efficiency were measured. The results are shown in Table 1,
[0196] Table 1 liposome particle size and encapsulation efficiency assay result
[0197]
[0198] The experimental results show that triptolide fatty acid esters can be prepared into liposomes under the same prescription conditions, the particle size distribution is 100-130nm, and the encapsulation efficiency is greater than 90%; The body cannot be prepared effectively, and the encapsulation efficiency is less than 50%. At the same time, a comprehensive comparison found that triptolide fatty acid modified by saturated fatty acid with carbon number ≥ 8 has smaller particle size and more uniform dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com