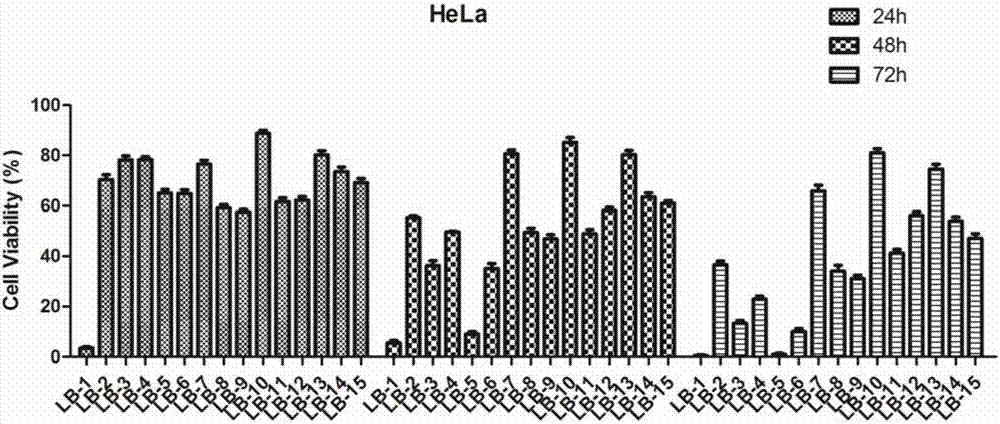
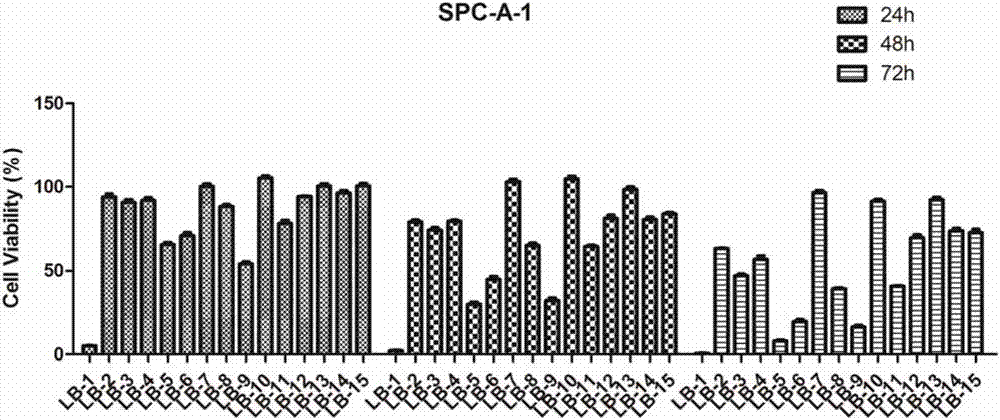
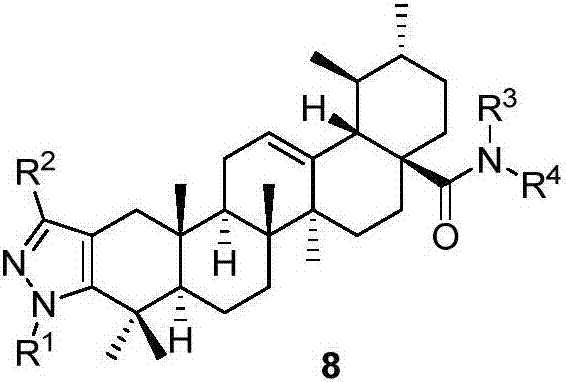
A class of pyrazole heterocycle-containing ursolic amide derivatives, synthesis and applications thereof
A synthesis method and compound technology, which can be applied to medical preparations containing active ingredients, drug combinations, steroids, etc., and can solve the problems of limited development, poor solubility, and low bioavailability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] Dissolve 460mg of ursolic acid (1) and 280mg of anhydrous potassium carbonate in 10ml of N,N-dimethylamide, then add 300mg of BnBr, and heat the reaction mixture at about 60°C for 4 hours until the reaction is complete. The mixture was cooled to room temperature, and 50 mL of H 2 O, a white solid was precipitated. The obtained solid was successively filtered, washed with water, and vacuum-dried to obtain 0.50 g of intermediate (2), and the yield of intermediate (2) was 92%.
[0126] The spectrum identification result of intermediate (2) is as follows:
[0127] 1 H NMR (500MHz, CDCl 3 ):δ=7.36-7.29(m,5H),5.24-5.23(m,1H),5.10(d,J=12.5Hz,1H),4.98(d,J=12.5Hz,1H),3.20(dd, J=11.0,4.5Hz,1H),2.04-1.98(m,1H),1.89-1.76(m,3H),1.73-1.67(m,2H),1.64-1.55(m,4H),1.53-1.44( m,6H),1.37-1.26(m,6H),1.07(s,3H),1.05-1.02(m,2H),0.98(s,3H),0.93(d,J=6.0Hz,3H),0.89 (s,3H),0.85(d,J=6.5Hz,3H),0.78(s,3H),0.64(s,3H).
[0128] 13 C NMR (125MHz, CDCl 3 ):δ=177.3,138.1,136.3,128.4(2C),128.1(2C...
Embodiment 2
[0132] Dissolve 450 mg of intermediate (2) in 50 mL of anhydrous dichloromethane, cool to below 0°C, add 355 mg of PCC for reaction, and slowly warm the mixture to room temperature and stir for about 12 hours until the reaction is complete. The reaction mixture was filtered through diatomaceous earth to remove insoluble matter, the organic phase was concentrated under pressure, and the residue was separated and purified by silica gel column chromatography to obtain 375 mg of intermediate (3). Intermediate (3) was a white solid. The yield was 84%.
[0133] The spectrum identification result of intermediate (3) is as follows:
[0134] 1 H NMR (500MHz, CDCl 3 ):δ=7.37-7.29(m,5H),5.25(t,J=3.5Hz,1H),5.11(d,J=12.5Hz,1H),4.99(d,J=12.5Hz,1H),2.57 -2.50(m,1H),2.40-2.34(m,1H),2.27(d,J=11.5Hz,1H),2.04-1.98(m,1H),1.92-1.87(m,3H),1.82-1.68 (m,3H),1.64-1.54(m,3H),1.50-1.41(m,5H),1.35-1.28(m,4H),1.10-1.07(m,1H),1.08(s,6H),1.03 (d,J=9.0Hz,6H),0.93(d,J=6.0Hz,3H),0.85(d,J=6.5Hz,3H),0.68(s,...
Embodiment 3
[0139] 300 mg of intermediate (3) was dissolved in 20 mL of anhydrous tetrahydrofuran, the reaction solution was cooled to 0°C, 45 mg of sodium methoxide was added thereto, and then 45 mg of ethyl formate was added. The reaction mixture was stirred at room temperature for about 4 hours until the reaction was complete. A small amount of water was added to quench the reaction and the mixture was extracted three times with ethyl acetate (3 x 30ml). The combined organic phases were sequentially washed with water and brine, dried over anhydrous sodium sulfate, and filtered. The organic phase was concentrated, and the residue was quickly separated by silica gel column chromatography to obtain intermediate (4a).
[0140] The spectrum identification results of intermediate (4a) are as follows:
[0141] 1 H NMR (500MHz, CDCl 3 ): δ=14.92(br s,1H), 8.57(s,1H), 7.37-7.30(m,5H), 5.28(t,J=3.3Hz,1H), 5.12(d,J=12.5Hz,1H ),4.99(d,J=12.5,1H),2.32-2.28(m,2H),2.05-1.92(m,4H),1.82-1.69(m,3H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com