Aromatic amine derivatives, preparation method, and application thereof
A technology of aromatic amine derivatives and aryl groups, which is applied in the field of aromatic amine derivatives and their preparation, can solve the problems of lifespan and luminous efficiency constraints of OLED devices, reduce surface plasmon effect, improve transmittance, and increase luminous efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
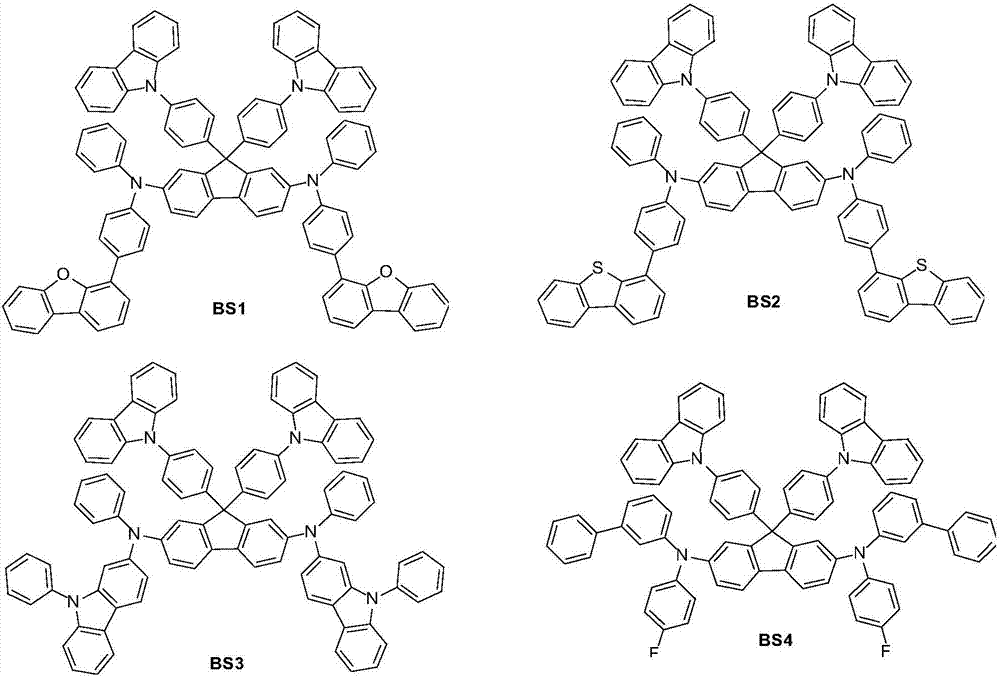
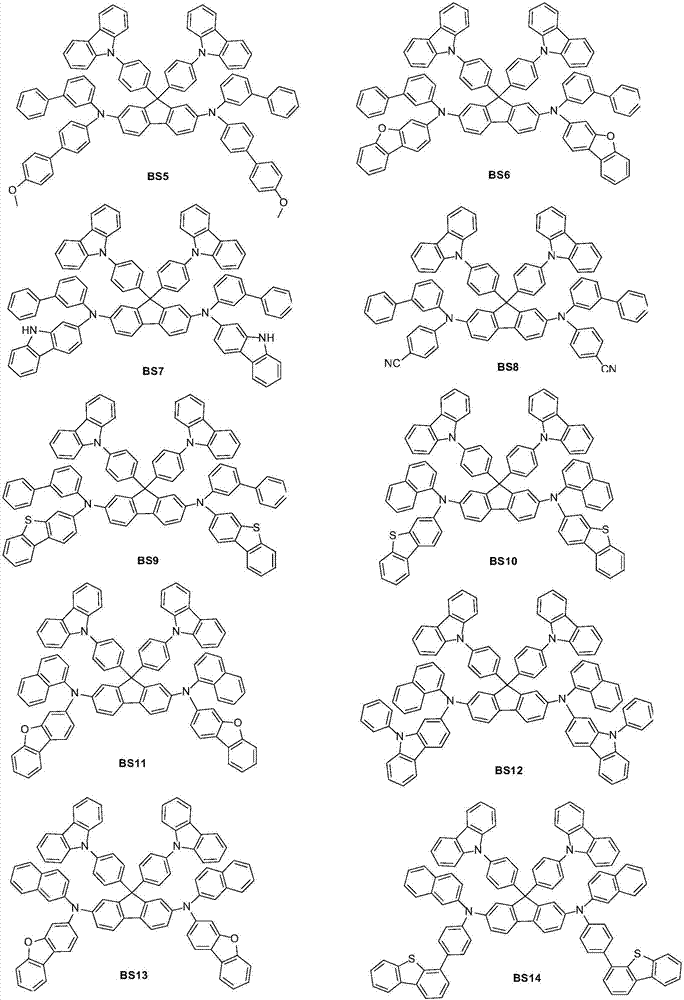
Examples
preparation example Construction
[0037] The present invention also provides a preparation method of the aromatic amine derivative, comprising:
[0038] The intermediate shown in formula (A) is reacted with the compound shown in formula (HO) to obtain the intermediate shown in formula (B):
[0039]
[0040] The intermediate shown in formula (B) is reacted with the bromide shown in formula (HT) again, obtains the aromatic amine derivative shown in formula (I):
[0041]
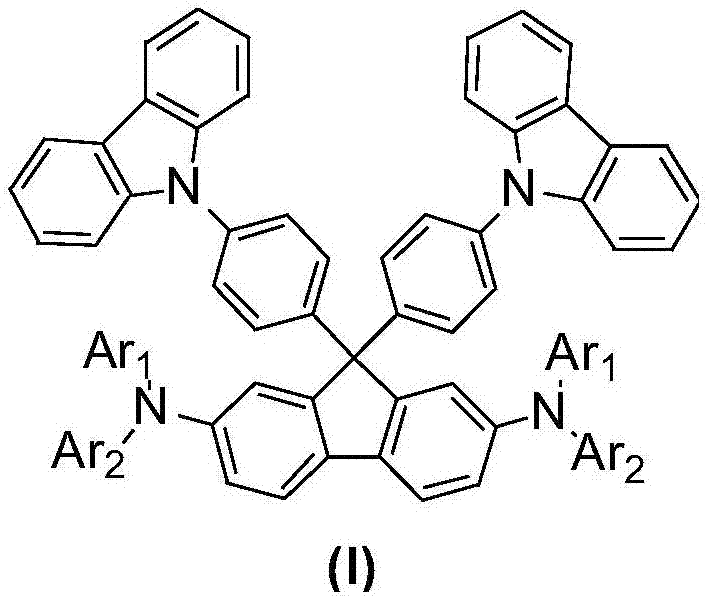
[0042] Among them, Ar 1 It is a C6-C18 aryl group, or a C10-C20 condensed aryl group with or without heteroatoms, Ar 2 It is a C6-C24 aryl group containing or not containing a heteroatom, or a C10-C20 condensed aryl group containing or not containing a heteroatom.
[0043] According to the present invention, the intermediate shown in formula (A) is prepared according to the method shown below:
[0044] Intermediate (A) is obtained by reacting 2,7-dibromo-9H-fluorenone (compound 1) with 9-phenyl-9H-carbazole (compound 2).
[0045]
...
Embodiment 1
[0057] Embodiment 1: the preparation of intermediate A
[0058] Under argon protection, 3.38g (10mmol) of 2,7-dibromo-9H-fluorenone (compound 1), 34g (140mmol) of 9-phenyl-9H- Carbazole (compound 2) and 0.96 g (10 mmol) of methanesulfonic acid were stirred at 140° C. for 6 hours. After the reaction, it was cooled to room temperature, extracted with dichloromethane, washed with saturated sodium bicarbonate solution and distilled water respectively, and the organic layer was concentrated to obtain a blue solid. The crude product was purified through a silica gel column using petroleum ether / dichloromethane (3:1) as eluent, and then recrystallized with acetone to obtain 6.29 g of intermediate A with a yield of 78%.
Embodiment 2
[0059] Embodiment 2: the preparation of intermediate B-1
[0060]
[0061] Under an argon atmosphere, 250 ml of dehydrated toluene was added to 40.33 g (50 mmol) of intermediate A, 9.31 g (100 mmol) of aniline, and 19.2 g (200 mmol) of sodium tert-butoxide, followed by stirring. 450 mg (2 mmol) of palladium acetate and 404 mg (2 mmol) of tri-tert-butylphosphine were added and reacted at 80° C. for 8 hours. After the reaction, it was cooled to room temperature, filtered through celite / silica gel, and the filtrate was concentrated under reduced pressure. The resulting residue was recrystallized from toluene, filtered, and dried to obtain 35.73 g of a solid, namely Intermediate B-1 (yield: 86%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Luminous efficiency | aaaaa | aaaaa |
| Luminous efficiency | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com