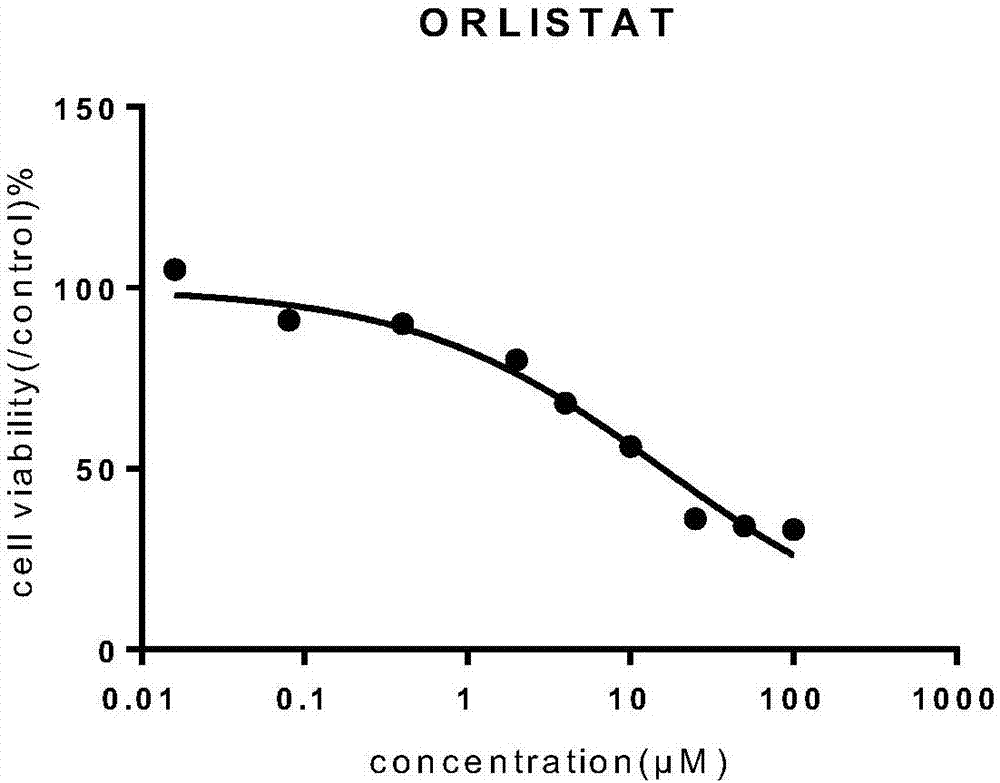
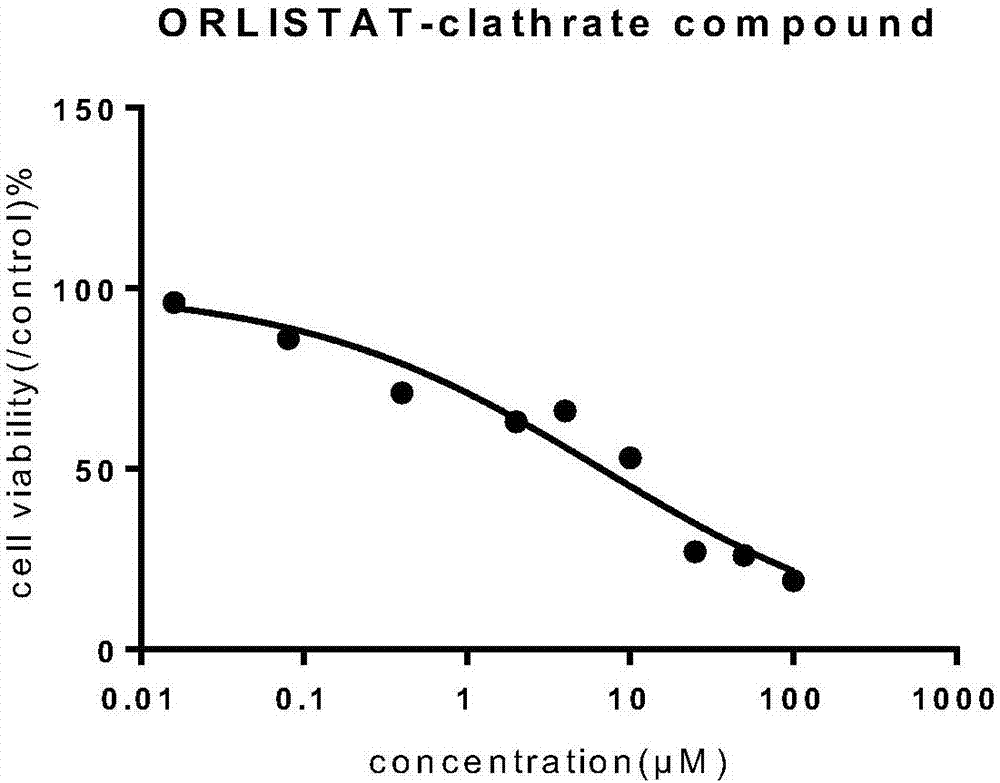
Orlistat inclusion compound and preparation method and application thereof in antineoplastic medicines
A technology of orlistat and cyclodextrin inclusion compound is applied to orlistat sulfobutyl ether-β-cyclodextrin inclusion compound and preparation thereof, and the application field of antitumor drugs can solve the problem of For the treatment and/or anti-tumor orlistat inclusion complex and other problems, to achieve the effects of good medicinal value, improved bioavailability, and good biosafety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Preparation of orlistat sulfobutyl ether-β-cyclodextrin inclusion compound
[0044] Preparation of orlistat sulfobutyl ether-β-cyclodextrin inclusion compound by grinding method comprises the following steps:
[0045] Take 10g of sulfobutyl ether-β-cyclodextrin, add it into 30mL of water medium, and grind it evenly; take 0.8g of orlistat and dissolve it in 2.4mL of ethanol to obtain an ethanol solution of orlistat; His ethanol solution is slowly added to the well-ground sulfobutyl ether-β-cyclodextrin, fully ground and then dried to obtain orlistat sulfobutyl ether-β-cyclodextrin inclusion compound.
Embodiment 2
[0046] Example 2 Preparation of orlistat sulfobutyl ether-β-cyclodextrin inclusion compound
[0047] Ultrasonic method prepares orlistat sulfobutyl ether-β-cyclodextrin inclusion compound, comprises the following steps:
[0048] Dissolve 25g of sulfobutyl ether-β-cyclodextrin in water medium to make a saturated solution; dissolve 1g of orlistat in acetone, slowly add it to the saturated solution of sulfobutyl ether-β-cyclodextrin, mix well After homogenization, ultrasonic treatment was performed for 6 hours, filtered, washed and dried to obtain orlistat sulfobutyl ether-β-cyclodextrin inclusion compound.
Embodiment 3
[0057] Example 3 Preparation of orlistat sulfobutyl ether-β-cyclodextrin inclusion compound
[0058] Preparation of orlistat sulfobutyl ether-β-cyclodextrin inclusion compound by grinding method comprises the following steps:
[0059] Take 10g of sulfobutyl ether-β-cyclodextrin, add it to 30mL of water medium, and grind it evenly; take 5g of orlistat and dissolve it in 15mL of ethanol to obtain an ethanol solution of orlistat; The ethanol solution is slowly added to the well-ground sulfobutyl ether-β-cyclodextrin, thoroughly ground and then dried to obtain the orlistat sulfobutyl ether-β-cyclodextrin inclusion compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com