Method for detecting related substances of ibuprofen and its sodium salt and preparation
A related substance, ibuprofen sodium technology, which is applied in the field of drug quality testing, can solve the problems of inability to achieve accurate qualitative and quantitative, rough inspection of impurities, etc., and achieve the effect of simple and easy method, simple method, accurate qualitative and quantitative
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
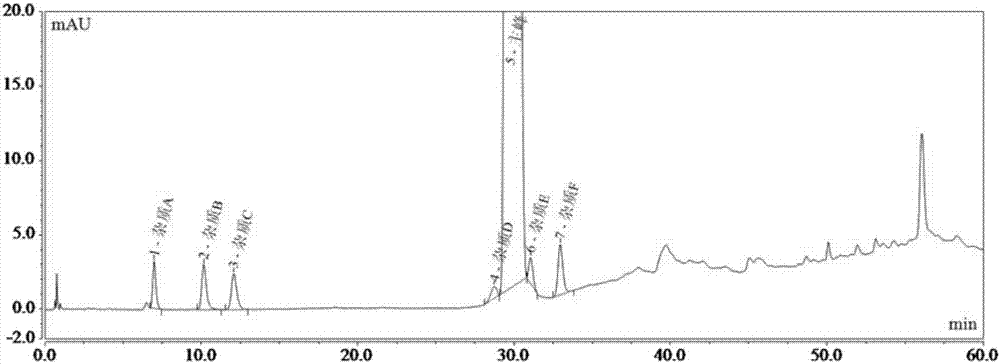
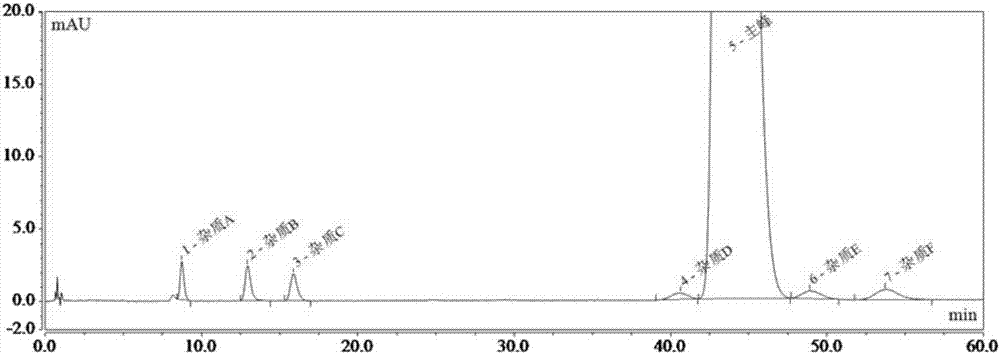
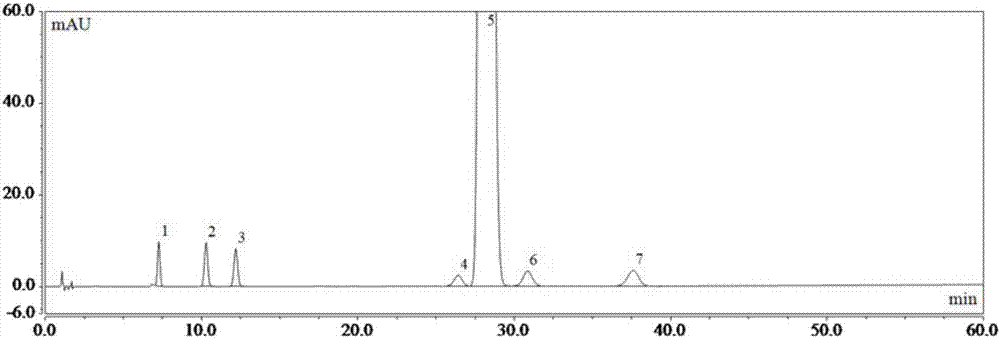
[0075] As previously mentioned, the present invention aims to propose a detection method for ibuprofen, its sodium salt and its preparation related substances A, B, C, D, E, F and G, to achieve effective detection between each impurity chromatographic peak. Separation (separation degree greater than 1.5), and accurate qualitative and quantitative impurities in ibuprofen, its sodium salt and its preparations.
[0076] Reach the main instrument and reagent used of the present invention object of the present invention as follows:
[0077] DV215CD electronic balance (US company OHAUS); Dionex Ultimate 3000 high-performance liquid chromatography (US company Dionex); Shimadzu LC-15C Shimadzu high-performance liquid chromatography (Shimadzu Instruments (Suzhou) Co., Ltd.).
[0078] Phosphoric acid (analytical grade, Shanghai Lingfeng Chemical Reagent Co., Ltd.); acetonitrile (chromatographic grade, American Tedia Company); n-hexane (chromatographic grade, Tianjin Concord Technology C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com