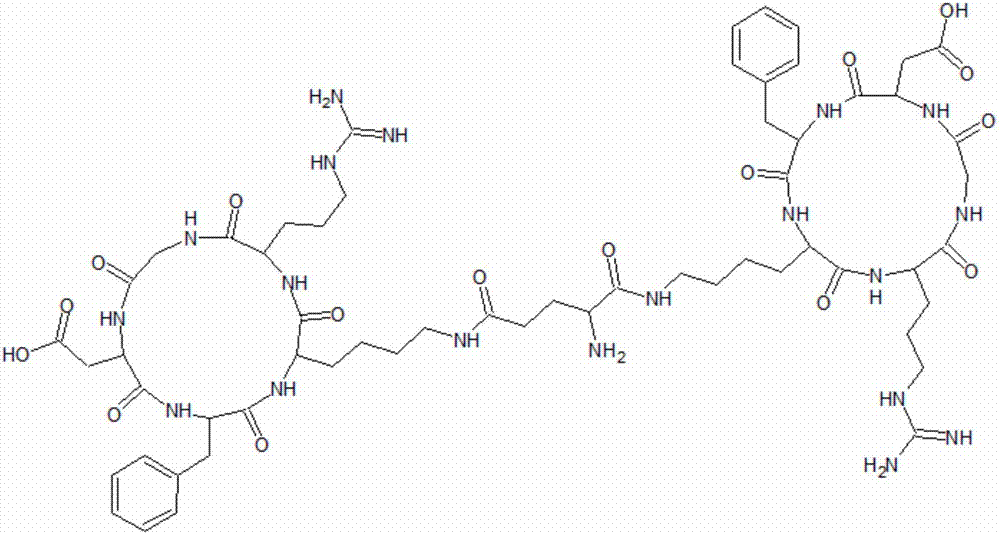
Biscyclopeptide E(c(RGDfK))2 and preparation method thereof
A bicyclic peptide, fmoc-l- technology, applied in the field of cyclic peptides, can solve problems such as poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
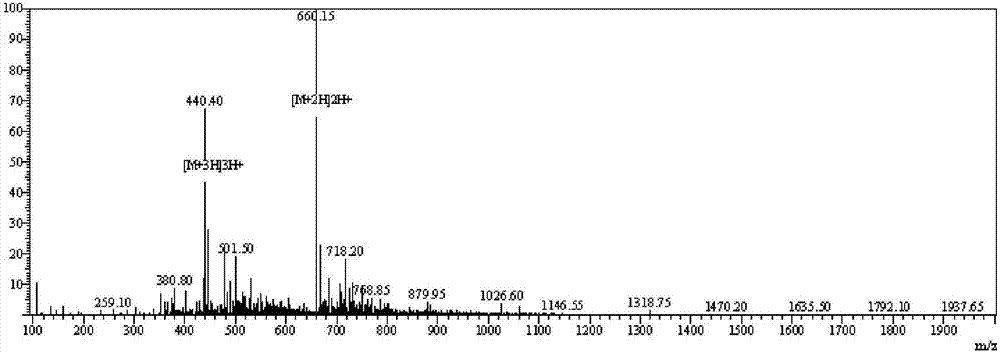
Image
Examples
preparation example Construction
[0025] The invention provides a bicyclic peptide E[c(RGDfK)] 2 The preparation method, this preparation method comprises:
[0026] 1) Soak the resin and Fmoc-Asp-oall (Fmoc-L-aspartic acid alpha-allyl ester) in DCM (dichloromethane), then add DIEA (N,N-diisopropylethylamine) Perform a contact reaction followed by washing;
[0027] 2) Add DCM, methanol and DIEA to the reaction system for capping treatment, then add piperidine for deprotection, and then wash until the reaction system is detected as blue by ninhydrin;
[0028] 3) G (glycine), HoBt (1-hydroxybenzotriazole), TBTU (O-benzotriazole-N, N, N', N'-tetramethylurea tetrafluoroborate) Add to the reaction system for contact reaction, and then wash until the reaction system is detected as blue by ninhydrin;
[0029] 4) Add R (arginine), K (lysine), and f (phenylalanine) to the reaction system in turn for contact reaction, and then add Pd (PPh 3 ) 4 (Tetrakis(triphenylphosphine)palladium) to carry out propenyl removal re...
Embodiment 1
[0056] Preparation of Bicyclic Peptide E[c(RGDfK)] 2
[0057] a. Weigh 1g of 2-cl resin, soak it in 20mL of DCM at 20°C for 2min, then wash once with DMF and DCM; use 20mL of DCM as solvent, weigh 0.5g of Fmoc-Asp-oall, mix with 1mL of DIEA, 1g of 2-cl resin was reacted at 20°C for 2h, and then washed twice with DMF; capped with 20mL DCM+1mL methanol+1mL DIEA at 20°C for 30min, washed three times with DMF; desorbed with 15mL piperidine at 20°C Protected, reacted for 15 minutes, and then washed 4 times with DMF until ninhydrin was detected as blue;
[0058] b. Add 0.9gG and 0.5mg HoBt to the system, add 1g TBTU as catalyst, 20mL DMF as solvent, react at 20°C for 1h, then wash 3 times until ninhydrin is detected as blue; add 2g R, 1.6 g K, 1.2g f were reacted at 20°C for 45 minutes;
[0059] c, with 1.2g of Pd (PPh 3 ) 4 , react at 20°C for 2h to remove the propenyl group; deprotect with 15mL piperidine at 20°C for 15min, then wash with DMF, DCM, and methanol in sequence un...
Embodiment 2
[0062] Preparation of Bicyclic Peptide E[c(RGDfK)] 2
[0063] a. Weigh 1g of 2-cl resin, soak it in 30mL of DCM at 25°C for 5min, then wash once with DMF and DCM; use 30mL of DCM as solvent, weigh 0.6g of moc-Asp-oall, and 1.5mL of DIEA , 1g of 2-cl resin was reacted at 25°C for 3h, and then washed twice with DMF; capped with 30mL DCM+1.5ml methanol+1.5mL DIEA at 25°C for 50min, washed 3 times with DMF; washed with 20mL piperidine at 25 Deprotect at ℃, react for 30 minutes, then wash with DMF 4 times until ninhydrin is detected as blue;
[0064] b. Add 1gG and 0.6g HoBt to the system, add 1.5g TBTU as catalyst, 30mL DMF as solvent, react at 25°C for 1.5h, then wash 3 times until ninhydrin is detected as blue; add 2.5g R, 2g K, 1.5g f were reacted at 25°C for 1h;
[0065] c, with 1.5g of Pd (PPh 3 ) 4, react at 25°C for 3h to remove the propenyl group; use 20mL piperidine to deprotect at 25°C for 30min, then wash with DMF, DCM, and methanol in sequence until the eluted was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com