Mononuclear magnesium cationization salt, its preparation method and application
A technology of magnesium cations and salts, applied in the direction of magnesium organic compounds, electrochemical generators, 2/12 group organic compounds without C-metal bonds, etc., can solve the problem of inability to achieve reversible deposition and dissolution, narrow chemical window and high activity Inability to use rechargeable magnesium batteries and other problems, to overcome the cumbersome synthesis method, high magnesium reversible deposition-dissolution efficiency, and overcome the effects of reversible deposition and dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
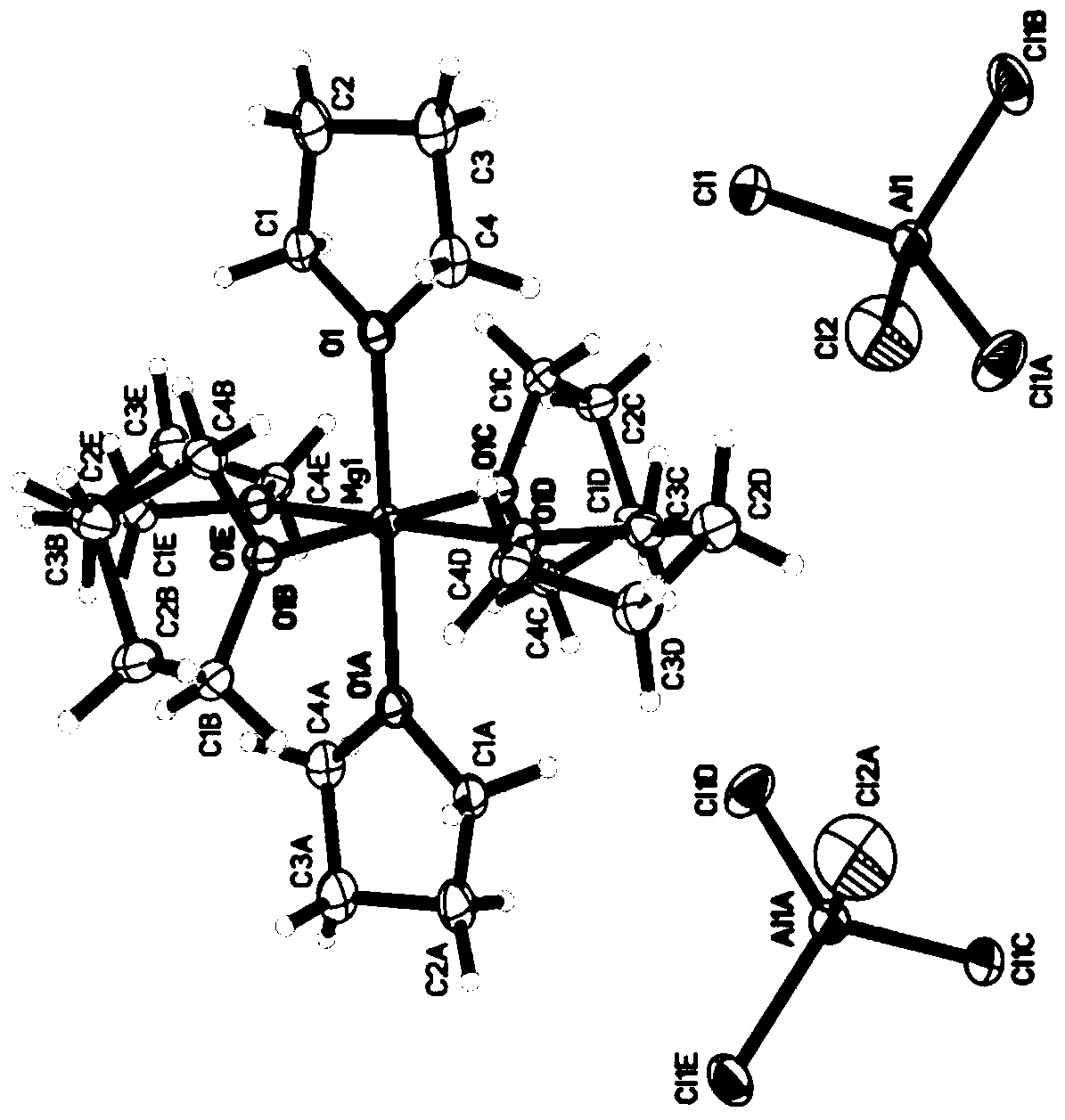
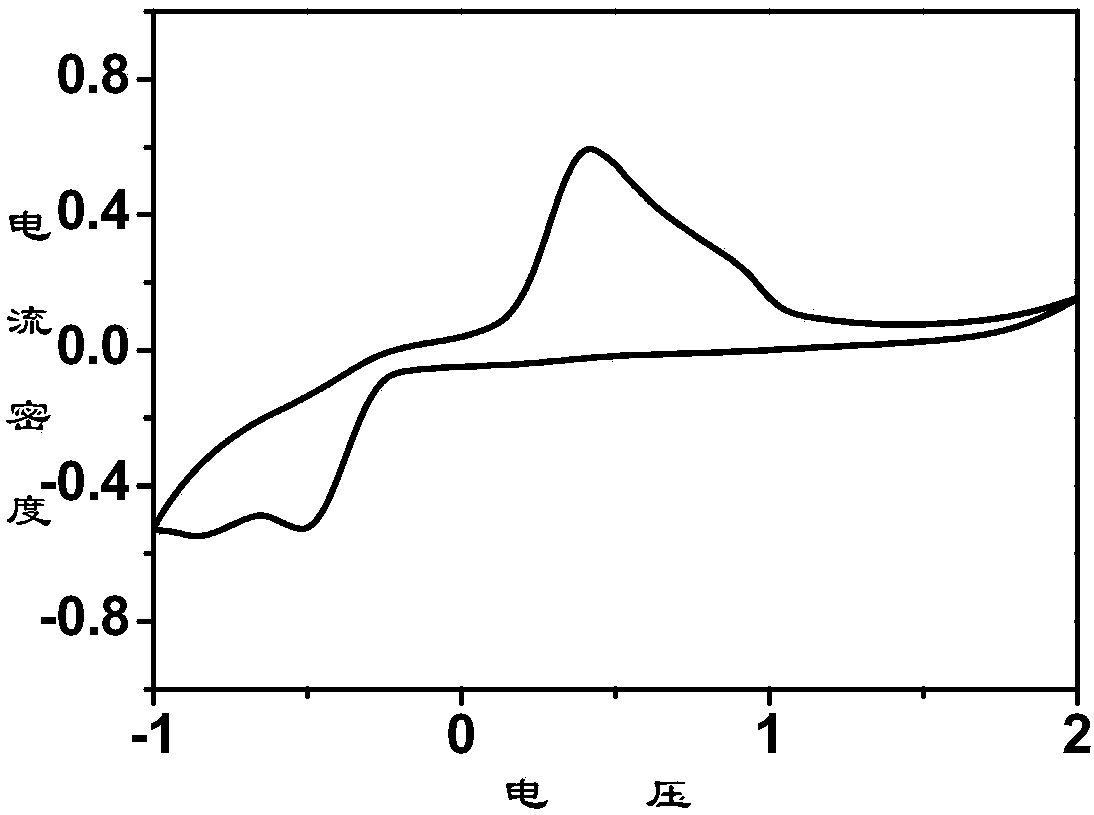
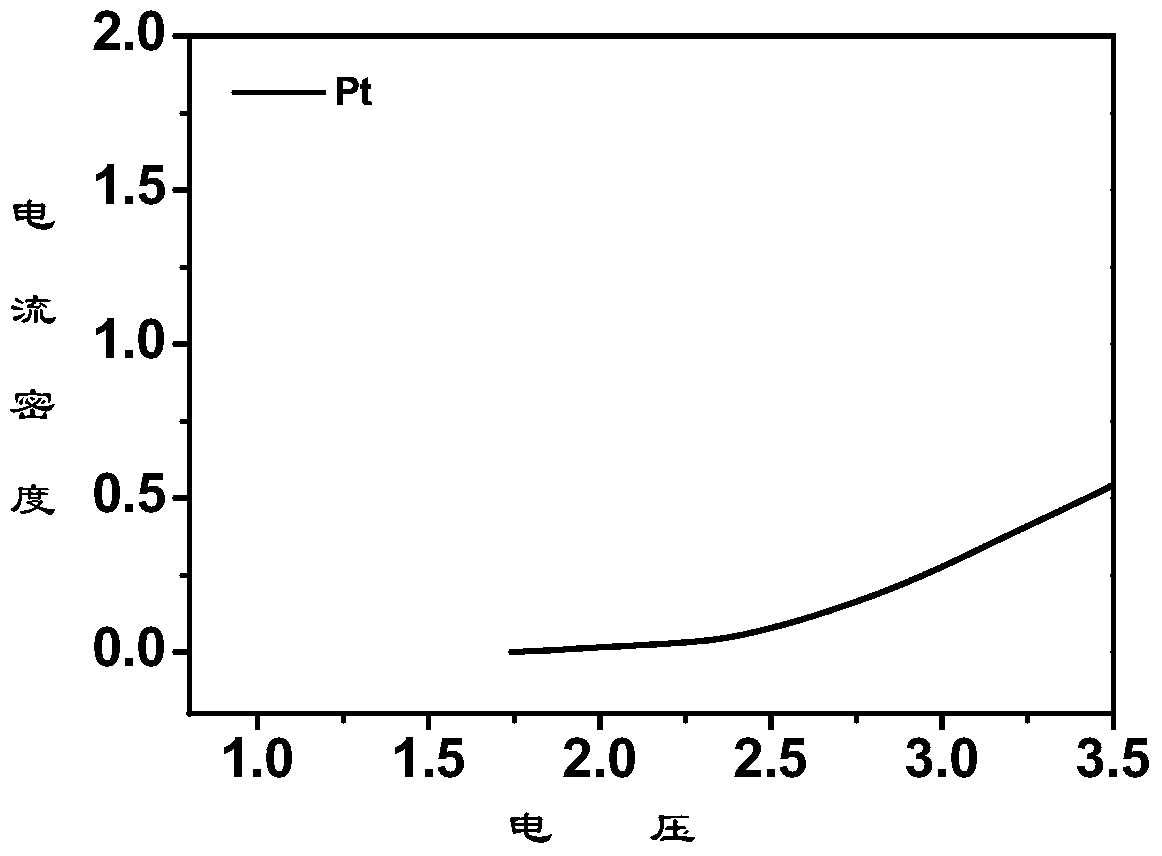
[0038] Embodiment 1: 56mg anhydrous magnesium chloride (MgCl 2 ) and 158mg of anhydrous aluminum chloride (AlCl 3 ) in 1 mL of ionic liquid 1-butyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide (n-methyl-(n-butyl)pyrrolidinium bis(trifluoromethanesulfonyl)imide, PYR14TFSI) at 95°C React for 24 hours to obtain a light yellow solution, cool to room temperature, and add 1mL THF to obtain a 0.3M rechargeable magnesium electrolyte. The crystal structure indicates that the electrolyte salt is [Mg(THF) 6 ][AlCl 4 ] 2 , whose crystal structure is as figure 1 shown. Elemental analysis theoretical value is C 36.28, H 6.09; measured value is C 36.27%, N 6.10%. Raman spectrum test result 350cm -1 anion AlCl 4 - Raman peaks of other aluminum chloride anions are not seen.
Embodiment 2
[0039] Embodiment 2: 56mg anhydrous magnesium chloride (MgCl 2 ) and 158mg of anhydrous aluminum chloride (AlCl 3 ) was reacted in 1mL triethylene glycol dimethyl ether (TEGDME) at 30°C for 24 hours to obtain a light yellow solution, cooled to room temperature to obtain a 0.6M rechargeable magnesium electrolyte, and the crystal structure indicated that the electrolyte salt was [Mg(TEGDME) 2 ][AlCl 4 ] 2 . Elemental analysis theoretical value is C 22.87, H4.48; measured value is C, 22.89%; N 4.47%. Raman spectrum test result 350cm -1 anion AlCl 4 - Raman peaks of other aluminum chloride anions were not seen.
Embodiment 3
[0040] Embodiment 3: 56mg anhydrous magnesium chloride (MgCl 2 ) and 158mg of anhydrous aluminum chloride (AlCl 3 ) in 1 mL of toluene at 100°C for 24 hours to obtain a light yellow solution, which was cooled to room temperature to obtain a 0.6M rechargeable magnesium electrolyte. The crystal structure of the electrolyte salt is [Mg(toluene) 6 ][AlCl 4 ] 2 . Elemental analysis theoretical value is C 55.15, H 5.29; measured value is C, 55.10%; H 5.30%. Raman spectrum test result 350cm -1 anion AlCl 4 - Raman peaks of other aluminum chloride anions were not seen.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com