Bipolar-configuration pyrenyl blue-light emitting material containing benzimidazole unit, preparation method and application
A technology of benzimidazole and blue light materials, which is applied in the direction of luminescent materials, chemical instruments and methods, electrical components, etc., can solve the problems of weakening the carrier transport performance between molecules, reducing device performance, and increasing the distance between molecules. Good electron transport ability, high decomposition temperature and glass transition temperature, high thermal stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1. Synthesis and property determination of compounds 3 and 4
[0037] 1. Synthetic route of compound 3 and 4
[0038] Synthesize compounds 3 and 4 according to the following reaction
[0039]
[0040] 1) Synthesis of 1,8-bis(4-tert-butylphenyl)pyrene (compound Py18)
[0041] Under the protection of nitrogen, 1,8-dibromopyrene (1.80g, 5mmol, 4-tert-butylphenylboronic acid (2.14g, 12mmol), tetrakis (triphenylphosphine) palladium Pd (PPh 3 ) 4 (0.12g, 0.1mmol) and 2M potassium carbonate solution (12mL) were dissolved in toluene (80mL) and refluxed for 24 hours. After the reaction, the mixture was poured into water, the organic layer was extracted with dichloromethane, and then with anhydrous MgSO 4 After drying, the solvent is removed by rotary evaporation. The crude product is loaded by dry method, and the mixture of n-hexane and dichloromethane is used as the eluent to pass through the silica gel column, and then the mixture of dichloromethane and ethanol (v:v=1:2) is r...
Embodiment 2
[0067] Example 2. Synthesis and property determination of compounds 9 and 10.
[0068] 1. Examples of synthetic routes of compounds 9 and 10:
[0069] Synthesize compounds 9 and 10 according to the following reaction
[0070]
[0071]
[0072] 1) Synthesis of 1-(4-phenyl)-1H-2-(3-bromophenyl)-benzimidazole (compound 3a)
[0073] A mixture of N-(4-phenyl)benzene-1,2-diamine (3.68g, 20mmol), 3-bromobenzaldehyde (3.70g, 20mmol) and sodium bisulfite (2.04g, 10mmol) was dissolved in DMF (80 mL) was stirred and refluxed in air for 1 h. After the reaction is finished, it is cooled to room temperature and the reaction liquid is poured into water to precipitate the product. After standing for a period of time, the product was filtered off with suction and washed with a small amount of methanol. Finally, the crude product is purified by silica gel column chromatography using a mixture of n-hexane and ethyl acetate (v:v=1:3) as an eluent to obtain a white powder solid product. Yield: 5.60 g,...
Embodiment 3
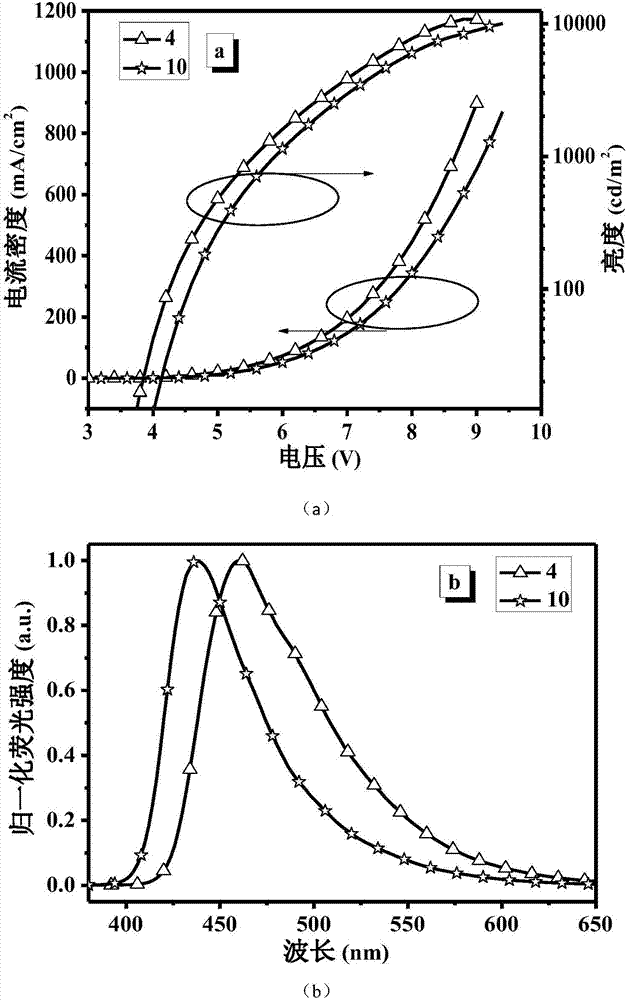
[0094] Example 3. Compounds 4 and 10 were selected to explore the electroluminescence properties of the materials, and organic electroluminescence devices were prepared by spin coating and thermal evaporation processes.
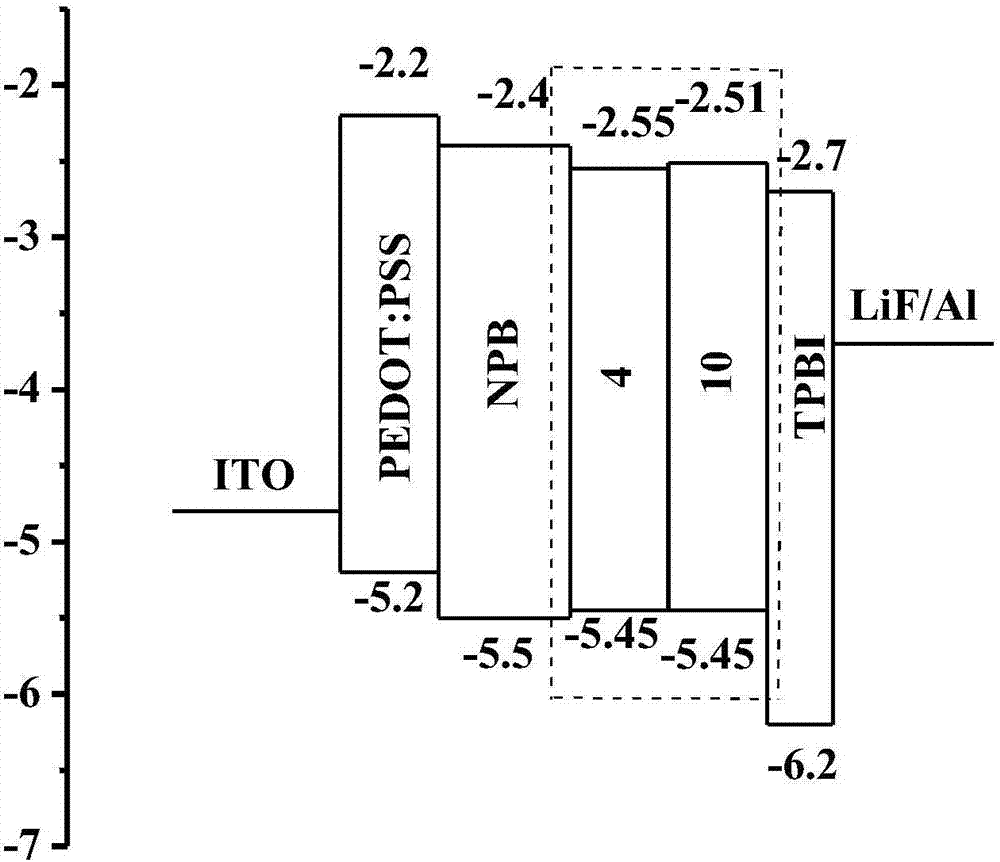
[0095] The structure of the organic electroluminescent device I is: ITO / PEDOT:PSS (40 nm) / NPB (30 nm) / 4or 10 (30 nm) / TPBI (20 nm) / LiF (1 nm) / Al (150 nm).
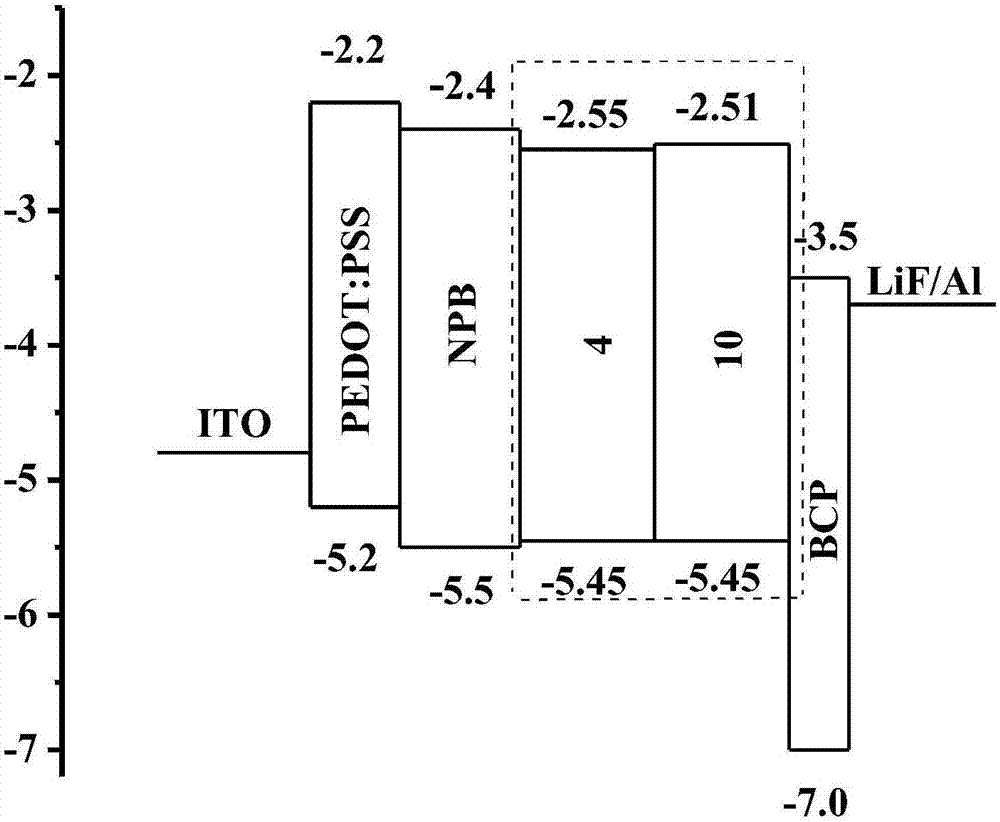
[0096] The structure of the organic electroluminescent device II is: ITO / PEDOT:PSS(40nm) / NPB(30nm) / 4or 10(30nm) / BCP(6nm) / LiF(1nm) / Al(150nm).
[0097] The substrate is made of glass sheet, the transparent conductive film is ITO film as the anode, the substrate is sequentially subjected to glass washing solution, deionized water cleaning, drying and ozone treatment, and then spin-coated a layer of 40nm thick polyethylene dioxythiophene-poly(benzene) Ethylene sulfonate) (PEDOT:PSS), and then dried in a drying oven at 120° for 30 minutes, and then placed in the vacuum chamber, when the vacuum reaches 4×10 –4 Pa unde...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com