Perylene diimide-rhodamine fluorescent probe, and preparation method and application thereof
A perylene diimide and fluorescent probe technology, applied in the field of fluorescent probes, can solve problems such as strong π-π interaction, application limitations of perylene diimide fluorescent compounds, poor solubility, etc., to achieve improved solubility, good Good solubility and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of perylenediimide-rhodamine fluorescent probe Rh-PDI
[0030] Rhodamine-Ethylamine (Rh-NH 2 ) was prepared according to the literature method (Xuan Zhang, Yasuhiro Shiraishi, Takayuki Hirai.Cu(II)-selective green fluorescence of a rhodamine-diacetic acid conjugate.Organic Letters, 2007,9(24),5040), the specific process is as follows: nitrogen protection Next, rhodamine B and ethylenediamine were refluxed in ethanol for 15h to obtain Rh-NH 2 .
[0031] 1,7-bis(4-tert-butylphenyloxy)-3,4,9,10-perylenetetracarboxylic dianhydride (PTA) was prepared by the following steps according to the literature method:
[0032] Step 1. Prepare 1,7-dibromo-3,4,9,10-perylenetetracarboxylic dianhydride according to literature method (Qiuli Zhao, Shuang Zhang, Yi Liu, Ju Mei, Sijie Chen, Ping Lu, Anjun Qin, Yuguang Ma, Jingzhi Sun, Benzhong Tang. Tetraphenylethenyl-modified perylene bisimide: aggregation-induced red emission, electrochemical properties and ordered microstruc...
Embodiment 2
[0047] UV Absorption and Fluorescence Properties of Perylene Diimide-Rhodamine Probe Rh-PDI in THF
[0048] Rh-PDI in tetrahydrofuran (the concentration of Rh-PDI is 10 -5 mol / L) UV-Vis absorption spectrum is a bimodal structure, the maximum absorption peaks are 500 and 530nm respectively.
[0049] The maximum wavelength of the fluorescence spectrum of Rh-PDI in tetrahydrofuran is located at 585nm, and the fluorescence is very weak. At this time, the rhodamine fluorophore in Rh-PDI is in the closed-loop "off" state and has no fluorescence; while the fluorescence of the perylene fluorophore in Rh-PDI is quenched due to photoinduced electron transfer, so Rh-PDI is very fluorescent in THF. weak.
Embodiment 3
[0051] Sensitive Response of Perylene Diimide-Rhodamine Probe Rh-PDI to pH
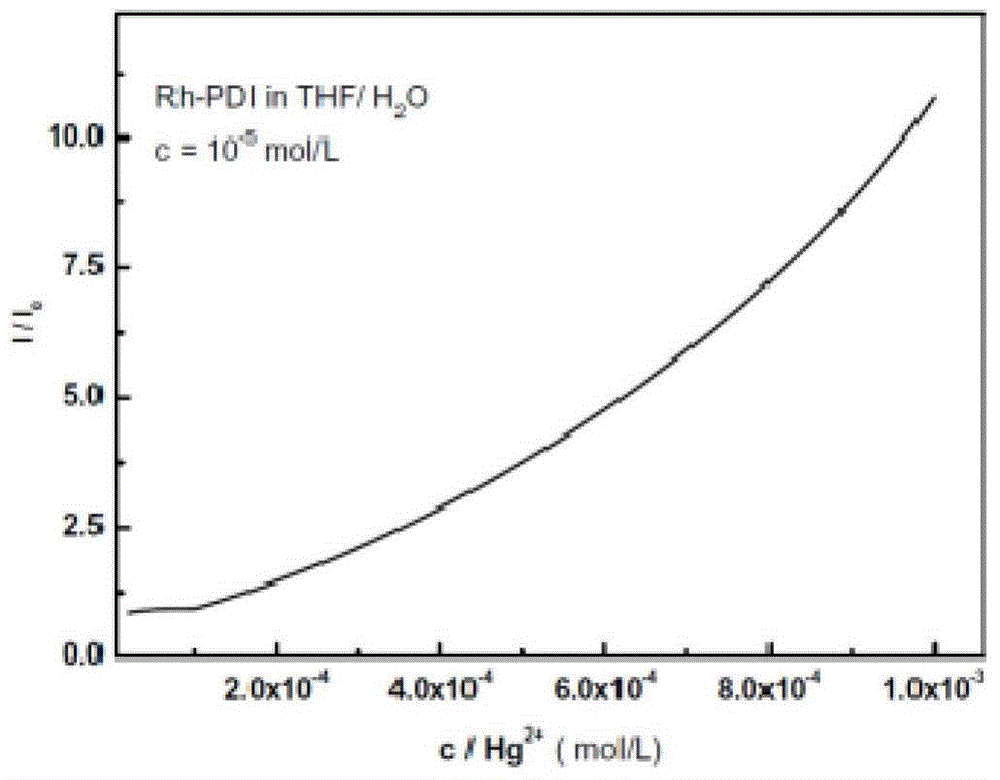
[0052] figure 1 It is the fluorescence spectrum of Rh-PDI in water / tetrahydrofuran mixed solution with pH response, wherein the concentration of Rh-PDI is 10 -5 mol / L. When the pH is greater than 2.2, the fluorescence of Rh-PDI is quenched in the water / THF mixed solution, and the perylenediimide-rhodamine fluorophore is in the "off" state. When the pH value is less than 2.2, under strong acidic conditions, Rh-PDI has a strong fluorescence in water / tetrahydrofuran mixed solution. From pH 2.17 to 1.38, the fluorescence of Rh-PDI increases rapidly, and the maximum emission peak is at 552nm, and the emission at 580nm The peak shoulder gradually increases. At this time, the perylenediimide-rhodamine fluorophore is in the "on" state. Show that Rh-PDI is a sensitive pH-responsive fluorescent probe.
[0053] With the pH response, the color of the probe molecules changed significantly under sunlight, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com