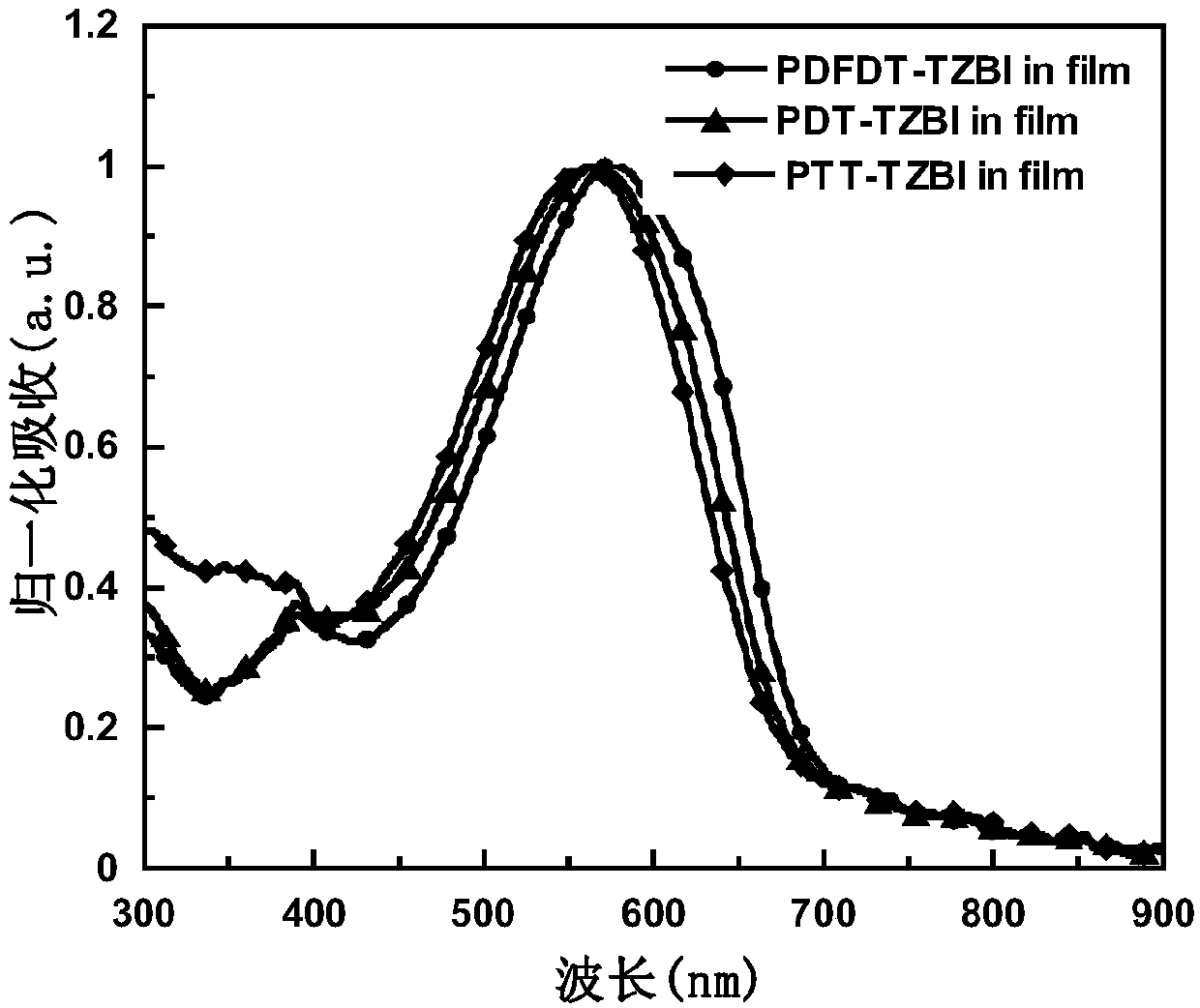
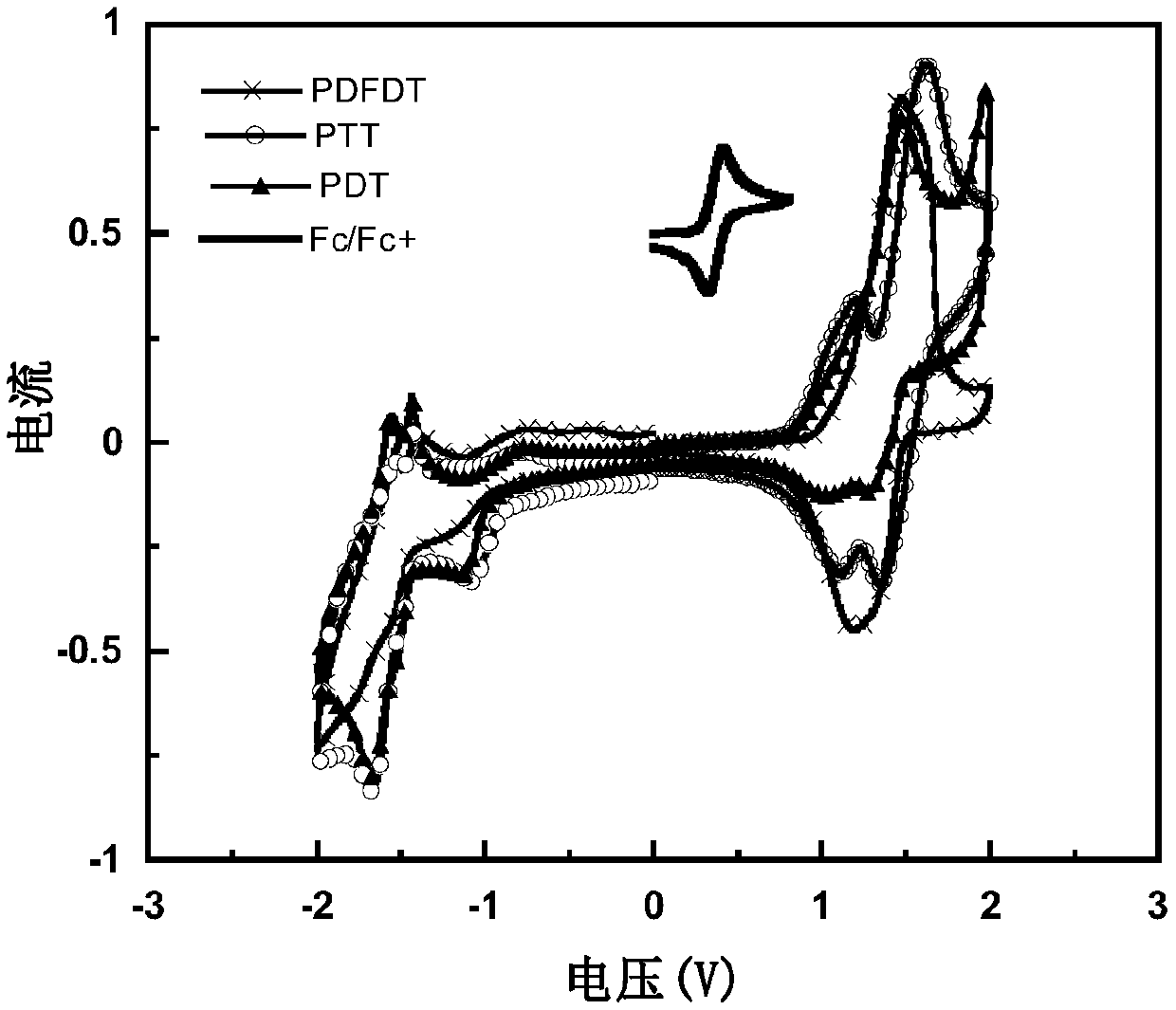
Conjugated polymer containing 1,2,3 triazoloisoindole-5,7(2h,6h)-dione and its preparation method and application
A conjugated polymer, isoindole technology, applied in the field of polymers, can solve problems such as insufficient species development, and achieve the effects of good solution processability, excellent optoelectronic properties, and improved solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 2,6-Dioctyl-4,8-bis(4-(2-octyldodecyl)thiophen-2-yl)-[1,2,3]triazolo[4,5-f] Preparation of isoindole-5,7)-dione (12):
[0039]
[0040] In a 500mL three-neck flask, under nitrogen protection, add compound 1 (13.83g, 41.66mmol), compound 2 (34.2g, 91.65mmol), then add 300mL tetrahydrofuran as a solvent, heat to 120 ° C, and determine the reaction process by spotting the plate Post-processing: Put the reaction mixture into a one-necked bottle and mix it with silica gel powder for rotary evaporation, then pass through a silica gel column for column purification to obtain a yellow oily liquid. Finally, 11.21 g of a yellow oily liquid is obtained. Yield: 83.23%. 1 H NMR (500MHz, CDCl 3 )δ7.33(d, J=1.4Hz, 2H), 7.15(d, J=1.3Hz, 2H), 2.56(d, J=6.8Hz, 4H), 1.61(d, J=5.9Hz, 2H) ,1.31-1.23(m,64H),0.88(t,J=7.0Hz,12H). 13 C (151MHz, CDCl3)
[0041] δ143.73, 136.68, 133.99, 132.80, 38.90, 33.26, 31.93, 31.92, 29.98, 29.67, 29.66, 29.63, 29.37, 29.34, 26.60, 22.70, 14.13.
[0...
Embodiment 2
[0061] 4,6-bis(5-bromo-4-(2-octyldodecyl)thiophen-2-yl)-2,6-dioctyl-[1,2,3]triazolo[4, Preparation of 5-f]isoindole-5,7(2H,6H)-dione:
[0062]
[0063] Add compound 12 (1.08g, 1.88mmol) into the reaction flask, add 80ml of chloroform to dissolve, then add 3ml of AcOH, weigh NBS (0.77g, 4.3mmol) into the reaction flask, and blow nitrogen. Cover the reaction apparatus with a black bag. Spot the plate to detect the progress of the reaction and end the reaction. Post-treatment: extract with DCM, and mix silica gel powder at the same time, purify the crude product with silica gel column, the volume ratio of petroleum ether:dichloromethane is 2:1. Finally, recrystallization was carried out with methanol and tetrahydrofuran. Finally, 4,6-bis(5-bromo-4-(2-octyldodecyl)thiophen-2-yl)-2,6-dioctyl-[1,2,3]triazolo[ 4,5-f]isoindole-5,7(2H,6H)-dione yellow oily liquid 1.2g, yield: 88%. 1 H NMR (500MHz, CDCl3) δ7.92(s, 2H), 4.76(t, J=7.2Hz, 2H), 3.83–3.52(m, 2H), 2.61(d, J=7.0Hz, 4H)...
Embodiment 3
[0065] Poly(5"-methyl-3-(2-octyldodecyl)-[2,2':5',2"-trithiophene]-5-yl)-8-(5-methyl- 4-octyldodecyl)thiophen-2-yl)-2,6-dioctyl-[1,2,3]triazolo[4,5-f]isoindole-5,7(2H , the preparation of 6H)-diketone, synthetic route and synthetic method are as follows:
[0066]
[0067] Under argon protection, in a 10mL microwave tube, add 4,8-bis(5-bromothien-2-yl)-6-octyl-5H-[1,2,5]selenadiazolo[3,4- f] Isoindole-5,7(6H)-dione (129mg, 0.10mmol), DT (49.2mg, 0.10mmol), tetrakis(triphenylphosphine)palladium (8mg) and xylene (2mL), Microwave at 200°C for 45 minutes, after the reaction has cooled to room temperature, precipitate the reaction solution in methanol, perform Soxhlet extraction with methanol, acetone, and n-hexane successively, and then add the aqueous solution of sodium diethyldithiocarbamate trihydrate (225mg, 1mmol, 100mL water) was stirred at 60°C for 8 hours, the palladium catalyst in the reaction was removed, Soxhlet extraction was carried out with methanol, acetone, n-h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com