Derivative containing alpha-aryl-alpha, beta-diamino acid ester and synthesis method and application thereof
A technology of a diamino acid ester and a synthetic method, applied in the field of synthetic medicine and chemical industry, can solve the problems of difficult large-scale application in industrialization, cumbersome operation and post-processing, limited economic value, etc., and achieves low cost, short reaction route, and preparation. short route effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108]
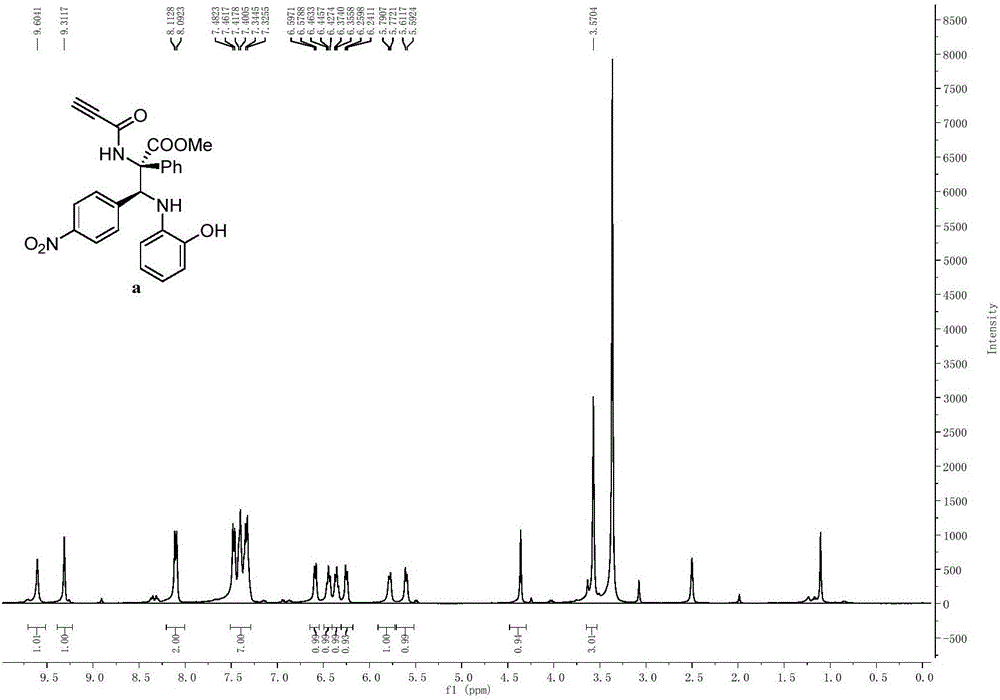
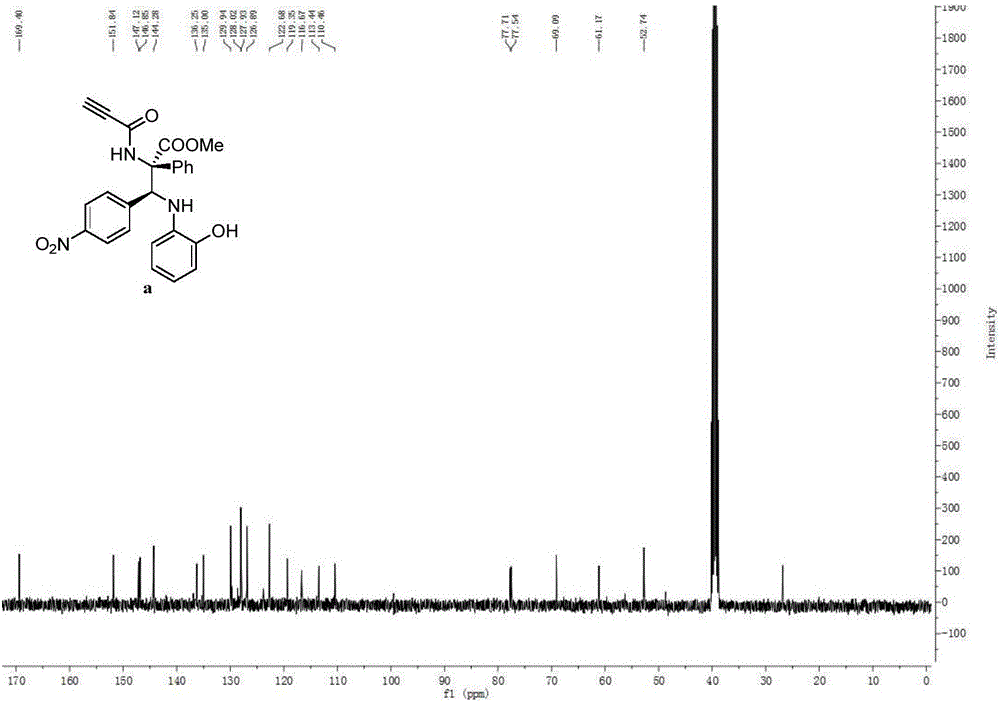
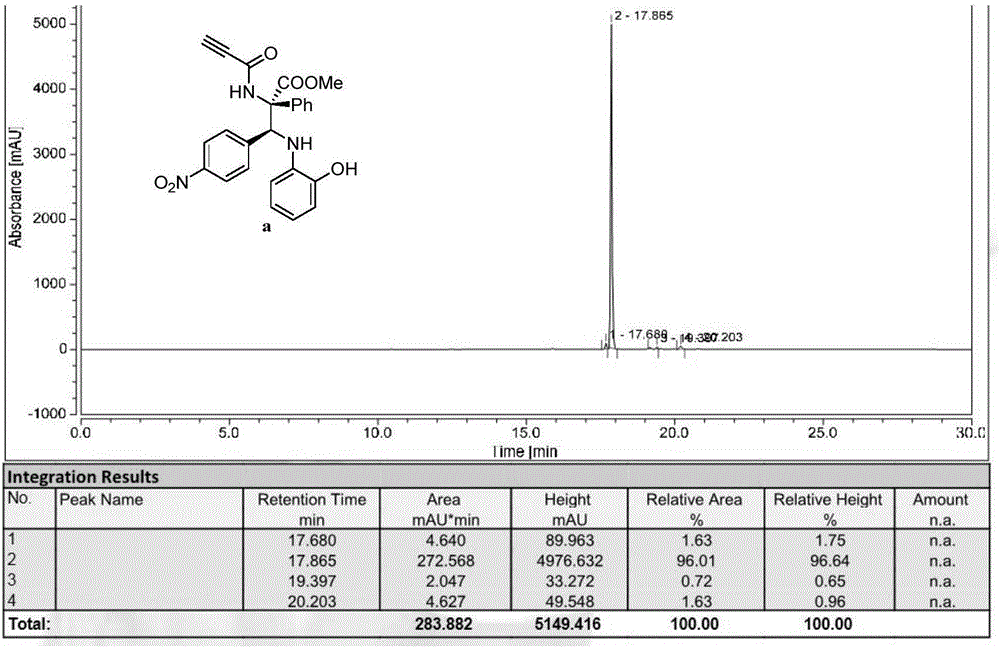
[0109] With imine (0.24mmol), rhodium acetate (0.0024mmol) and Molecular sieves (300mg) were mixed in a 10mL single-mouth bottle, the oil pump was used for nitrogen ventilation, and 1mL redistilled CH was added with a 1mL syringe. 2 Cl 2 Solution, prepared as mixed solution A, stirred at room temperature for 10 minutes. Then the aryl diazo compound (0.2mmol) and the amide compound (0.2mmol) were dissolved in 1 mL redistilled CH2 Cl 2 solution, prepared as Solution B. Solution B was added to mixed solution A with a syringe pump within 1 hour at 25°C. Stirring for 3 to 12 hours, the reaction mixture was purified by column chromatography to obtain a pure product, the structure of which was shown in formula (a), which was methyl(2S,3S)-3-(4-nitrophenyl)-3 -((2-Hydroxyphenyl)amino)-2-phenyl-2-propynamide propionic acid methyl ester, the yield is 87%, the dr value is equal to 89:11, and the HPLC purity is 96%. Compound shown in formula (a) 1 H NMR schematic as fi...
Embodiment 2
[0112]
[0113] With imine (0.24mmol), rhodium acetate (0.0024mmol) and Molecular sieves (300mg) were mixed in a 10mL single-mouth bottle, the oil pump was used for nitrogen ventilation, and 1mL redistilled CH was added with a 1mL syringe. 2 Cl 2 Solution, prepared as mixed solution A, stirred at room temperature for 10 minutes. Then the aryl diazo compound (0.2mmol) and the amide compound (0.2mmol) were dissolved in 1 mL redistilled CH 2 Cl 2 solution, prepared as Solution B. Solution B was added to mixed solution A with a syringe pump within 1 hour at 25°C. Stirring for 3 to 12 hours, the reaction mixture was purified by column chromatography to obtain a pure product, the structure of which was shown in formula (b), which was methyl(2S,3S)-3-(2-bromophenyl)-3- Methyl ((2-hydroxyphenyl)amino)-2-phenyl-2-propynamide propionate, yield 54%, dr value equal to 94:6, HPLC purity 95%. Compound shown in formula (b) 1 H NMR schematic as Figure 4 As shown, its 13 C NMR sc...
Embodiment 3
[0116]
[0117] With imine (0.24mmol), rhodium acetate (0.0024mmol) and Molecular sieves (300mg) were mixed in a 10mL single-mouth bottle, the oil pump was used for nitrogen ventilation, and 1mL redistilled CH was added with a 1mL syringe. 2 Cl 2 Solution, prepared as mixed solution A, stirred at room temperature for 10 minutes. Then the aryl diazo compound (0.2mmol) and the amide compound (0.2mmol) were dissolved in 1 mL redistilled CH 2 Cl 2 solution, prepared as Solution B. Solution B was added to mixed solution A with a syringe pump within 1 hour at 25°C. Stirring for 3 to 12 hours, the reaction mixture was purified by column chromatography to obtain a pure product, the structure of which was shown in formula (c), which was methyl (2S,3S)-3-(3-bromophenyl)-3- ((2-Hydroxyphenyl)amino)-2-phenyl-2-propynamide propionic acid methyl ester, the yield was 70%, the dr value was equal to 95:5, and the HPLC purity was 97%. Compound shown in formula (c) 1 H NMR schematic a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com