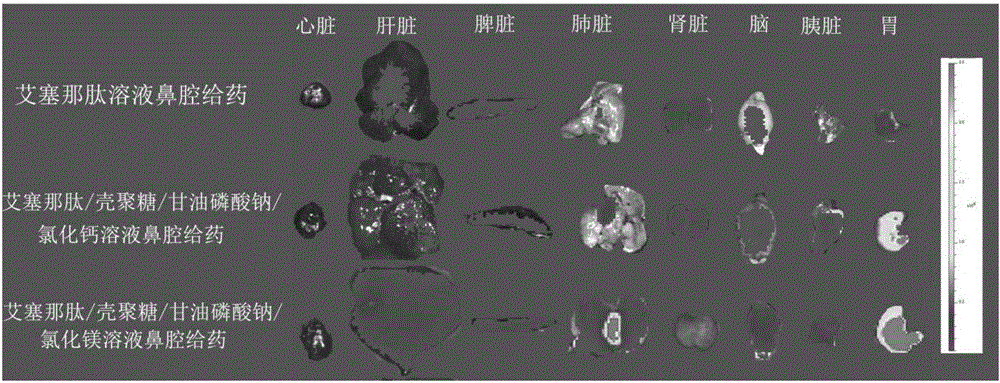
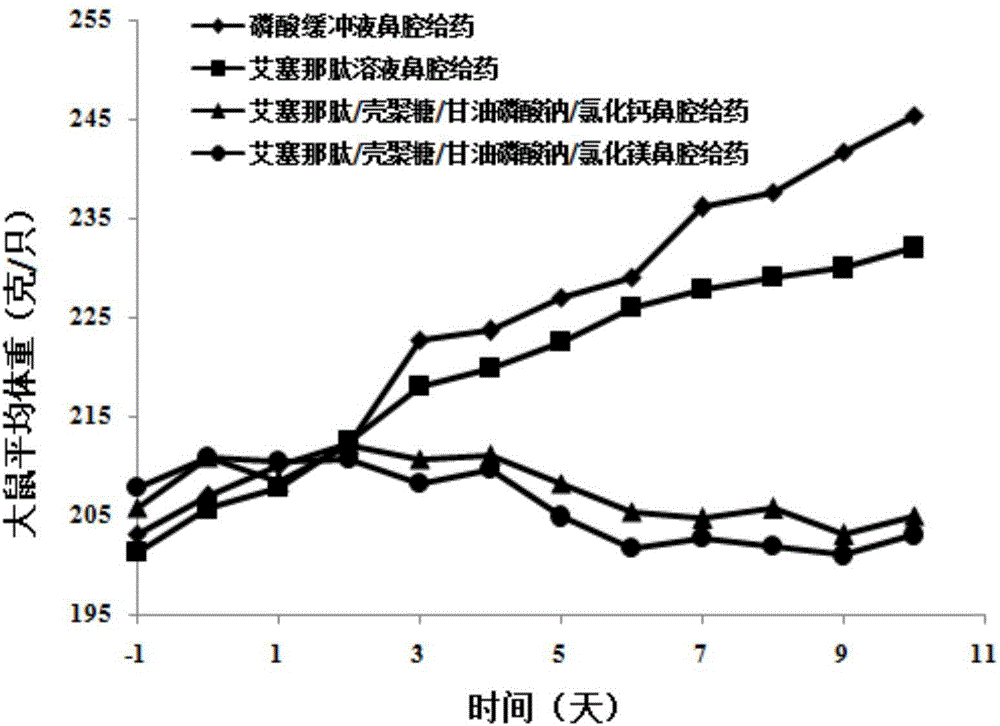
Exenatide nasal drug delivery preparation and preparation method thereof
A technology for nasal administration preparations and exenatide, which is applied in the field of pharmaceutical preparations and pharmaceutical engineering pharmaceutical preparations, can solve the problems of easy degradation and poor stability of exenatide, achieve weight loss, solve poor stability, improve Effects on quality of life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation of Exenatide Nasal Administration Preparation
[0028] Dissolve chitosan in 0.1 M lactic acid. After fully dissolving, add the mixed aqueous solution of inorganic salt and sodium glycerophosphate dropwise under ice bath conditions, stir at 200 rpm for 15 minutes, and mix well; then, add For exenatide, adjust the pH of the mixed solution to 4.0-6.0 with 0.1M sodium hydroxide and 0.1M hydrochloric acid, stir at 200 rpm for 15 minutes, mix well, and dispense into 1 ml EP tubes or vials. After pre-freezing at -80°C for 12 hours, vacuum freeze-drying with a lyophilizer for 12 hours to obtain a lyophilized powder preparation for nasal administration containing exenatide.
[0029] Viscosity measurement method: put the solution in a small beaker with an interlayer, keep the temperature of the solution in the beaker constant by controlling the temperature of the interlayer, and use the NDJ-8S rotational viscometer to measure the rotational viscosity of the ...
Embodiment 2
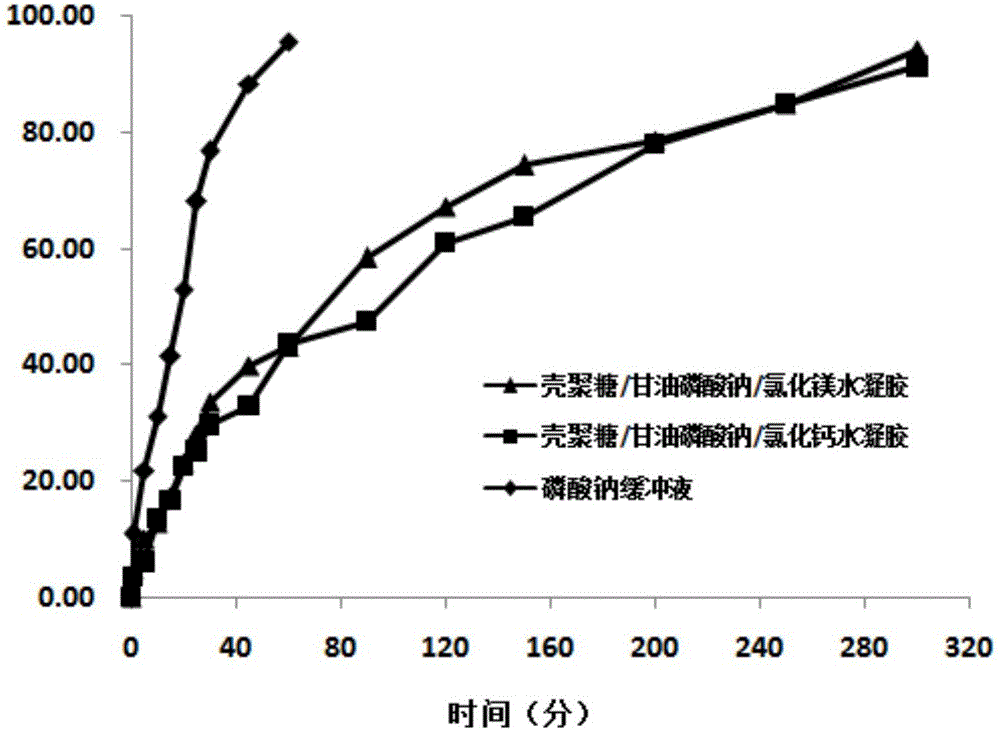
[0034] Dissolve chitosan (molecular weight: 80,000-120,000) in 0.1M lactic acid, add magnesium chloride / α,β-sodium glycerophosphate dropwise to it under ice bath conditions, adjust the pH to 6.0 with NaOH, 200 Stir at rpm for 15 minutes. The concentrations of chitosan, α,β-sodium glycerophosphate and magnesium chloride in the solution are shown in Table 1. The prepared chitosan / α,β-sodium glycerophosphate / magnesium chloride mixed solution is subpackaged, and freeze-dried powder is obtained after vacuum freeze-drying treatment, and deionized water is added to redissolve, and the volume of the solution is the same as that before freeze-drying. The influence of the concentration of each substance in the system on the resolubility of the lyophilized powder, the formation of hydrogel at 4 ℃, 20 ℃ and 37 ℃ and the long-term storage stability of the lyophilized powder were investigated.
[0035]The experimental results are shown in Table 1. Whether the chitosan / α,β-sodium glyceropho...
Embodiment 3
[0038] Dissolve chitosan in 0.1M lactic acid, add calcium chloride / α,β-sodium glycerophosphate dropwise to it in an ice bath, adjust the pH to 6.0 with NaOH, and stir at 100 rpm for 15 minutes . The concentrations of chitosan, α,β-sodium glycerophosphate and calcium chloride in the solution are shown in Table 2. The mixed chitosan / α,β-sodium glycerophosphate / calcium chloride mixed solution was subpackaged, and the freeze-dried powder was obtained by vacuum freeze-drying, and redissolved in deionized water. The volume of the solution was the same as before freeze-drying. The effect of the concentration of each substance in the lyophilized powder on the resolubility of the lyophilized powder, the formation of hydrogel at 4 ℃, 20 ℃ and 37 ℃, and the long-term storage stability of the lyophilized powder.
[0039] The experimental results are shown in Table 2. Whether the redissolution of the chitosan / α,β-sodium glycerophosphate / calcium chloride hydrogel system is affected by the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com