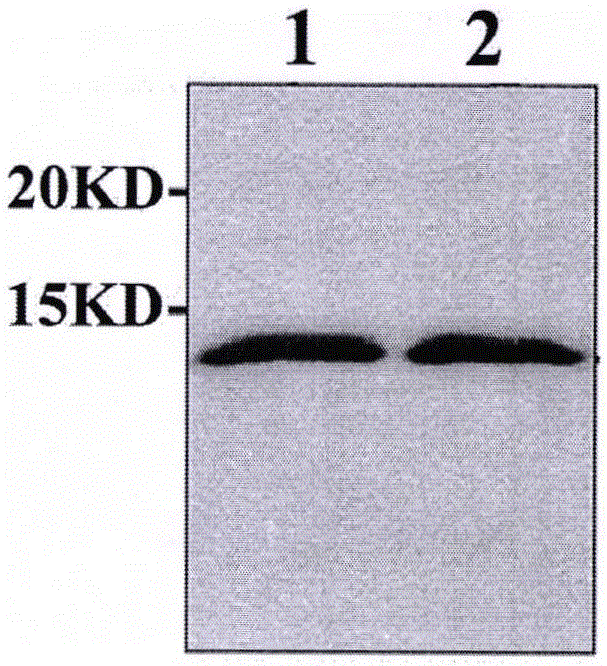
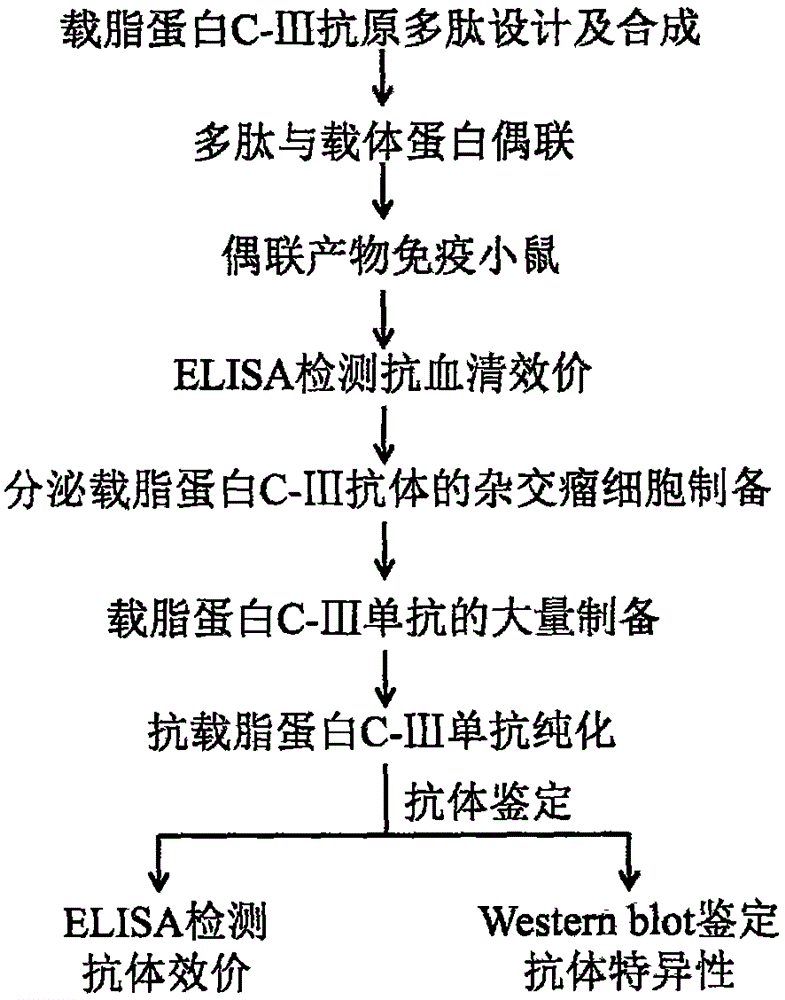
Preparation and application of apolipoprotein C-III monoclonal antibody
A technology of monoclonal antibody and apolipoprotein, applied in the field of bioengineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: Apolipoprotein C-III antigen polypeptide design
[0016] 1.1 According to the apolipoprotein C-III GenBank accession number GI: 4557323, obtain the human apolipoprotein C-III protein sequence (UniProt: P02656), which contains 79 amino acids.
[0017] 1.2 Use ProtParam (http: / / web.expasy.org / protparam / ) to analyze the characteristics of apolipoprotein C-III protein online. The analysis results are shown in Table 1. The protein has a molecular weight of 8.7646KD and an isoelectric point of 4.72, which is acidic protein.
[0018] Table 1 Analysis results of basic physicochemical properties of apolipoprotein C-III
[0019]
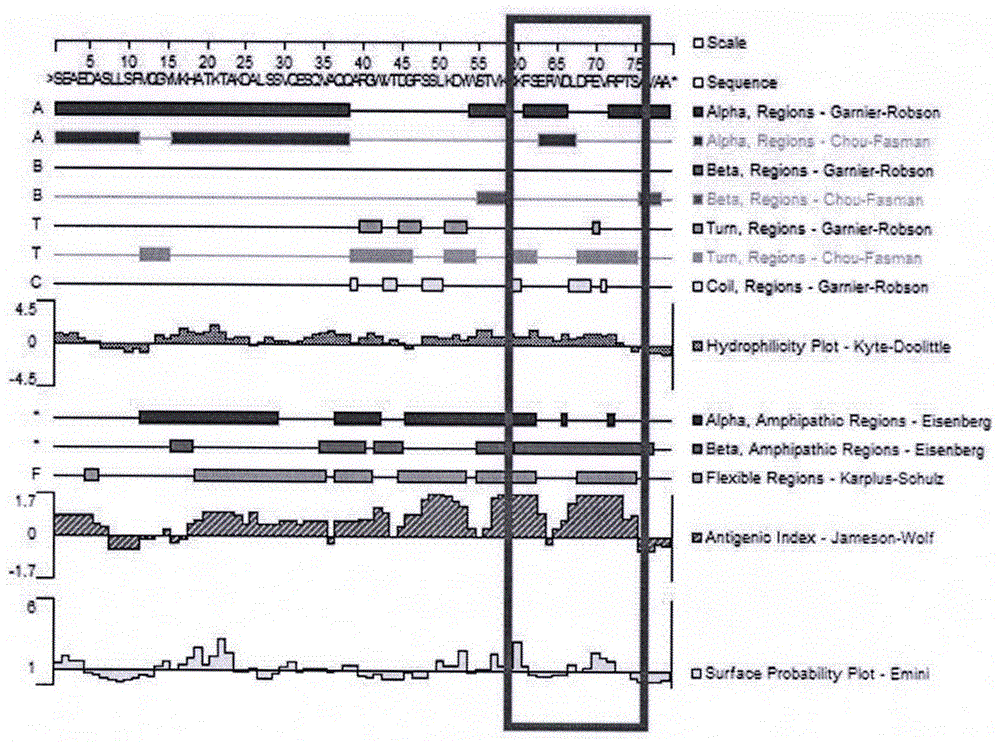
[0020] 1.3 DNAstar software was used to analyze the immunogenicity, hydrophilicity and surface accessibility of apolipoprotein C-III protein, and the results showed that there were 16 amino acids near the C-terminus of apolipoprotein C-III with antigenicity, hydrophilicity and surface accessibility stronger (see figure 1 ).
[0021]...
Embodiment 2
[0023] Example 2: Synthesis and coupling of apolipoprotein C-III antigen polypeptide
[0024] 2.1 Apolipoprotein C-III polypeptide synthesis
[0025] The peptide was synthesized by Shanghai Gil Biochemical Company with a purity of 93.85%.
[0026] 2.2 Coupling of polypeptide and carrier protein
[0027] Dissolve 5mg of polypeptide in 50μl DMSO, add coupling buffer to dilute to a final volume of 1ml, take out 200μl for ELISA detection, add 400μl of OVA solution with a concentration of 10mg / ml to the remaining 800μl of polypeptide solution and mix well, then add 200μl EDC with a concentration of 10 mg / ml was mixed immediately and reacted at room temperature for 2 hours.
[0028] 2.3 Ultrafiltration and packing of coupling products
[0029] Dilute the coupling product to 4ml with PBS, perform ultrafiltration on the coupling product with a 10KD Milipore ultrafiltration centrifuge tube, discard the filtrate in the lower tube after each ultrafiltration, and continue to use PBS wh...
Embodiment 3
[0030] Example 3: Preparation of hybridoma cells resistant to apolipoprotein C-III
[0031] 3.1 Immunization of animals
[0032] Select 6 clean grade BALB / c mice aged 6-8 weeks, take aliquoted coupling protein and equal volume of corresponding adjuvant to emulsify and mix the mice, and immunize the mice. The immunization procedure is shown in Table 2.
[0033] Table 2 Animal immunization program
[0034]
[0035] 3.2 Detection of serum antibody titer by ELISA method
[0036] 7 days after the third immunization, blood was taken from the tail of the immunized mice, and the serum antibody titer was detected by ELISA. The specific steps were as follows:
[0037] Coat the ELISA plate with synthetic apolipoprotein C-III polypeptide (2 μg / ml) as antigen, 100 μl per well, incubate overnight at 4 °C, discard the coating solution, add 200 μl 15% skimmed milk powder to each well, block at 37 °C for 1 h , washed 3 times, added serum samples diluted at a ratio of 1:1000, 1:2000, 1:40...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com