Vitamin B12 and BtuF protein interaction analysis method
A protein interaction and analysis method technology, which is applied in the analysis field of vitamin B12 and BtuF protein interaction, can solve the problems of protein molecular weight limitation, time-consuming map analysis, large sample volume, etc., to achieve less sample volume and sample purity The effect of low cost and simple operation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In the first step, BtuF protein and all protein standards (including alcohol dehydrogenase, concanavalin, avidin, β-lactoglobulin, myosin, cytochrome c, and serum amyloid) were prepared in 10kD The ultrafiltration membrane was desalted, the centrifugation temperature was 4°C, the centrifugation speed was 14000rcf, and the centrifugation was 30min, repeated 5 times, wherein the desalted BtuF protein was replaced with pH8. into the ammonium acetate buffer at pH 7.4;
[0025] In the second step, an equimolar amount of vitamin B12 solution prepared with an ammonium acetate buffer solution of pH 8.0 is added to the BtuF protein solution obtained in the first step to obtain a BtuF-VB complex solution;
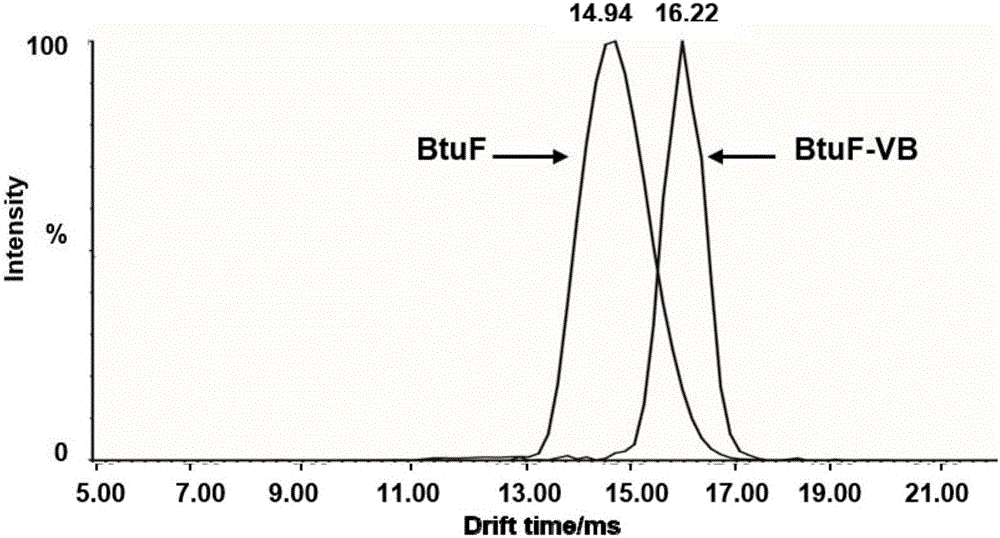
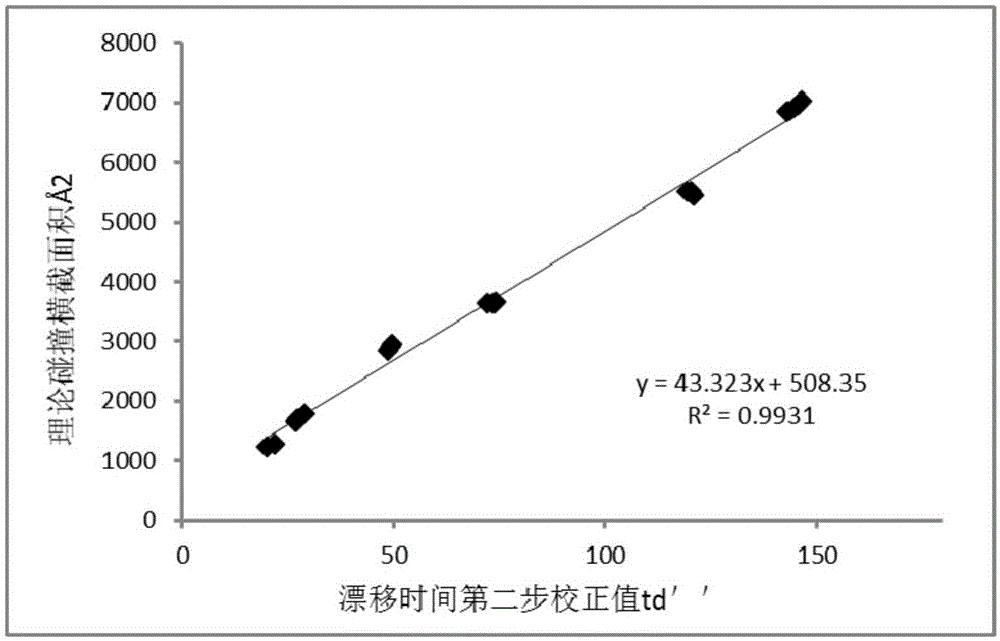
[0026] The third step is to draw 2 μL of BtuF protein and BtuF-VB with a final concentration of 10uM and a final concentration of 5uM protein standard for mass spectrometry, and adjust the instrument parameters such as: the working mode is ion mobility mode, the ionization mod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com