Method of preparing o-phenol compound by enzyme method
A technology for enzymatic preparation and compound application in biochemical equipment and methods, enzymes, immobilized enzymes, etc., which can solve the problems of high environmental pollution, poor specificity, complex reaction process, etc., and achieve high reaction specificity and cycle time. The effect of short and simple process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
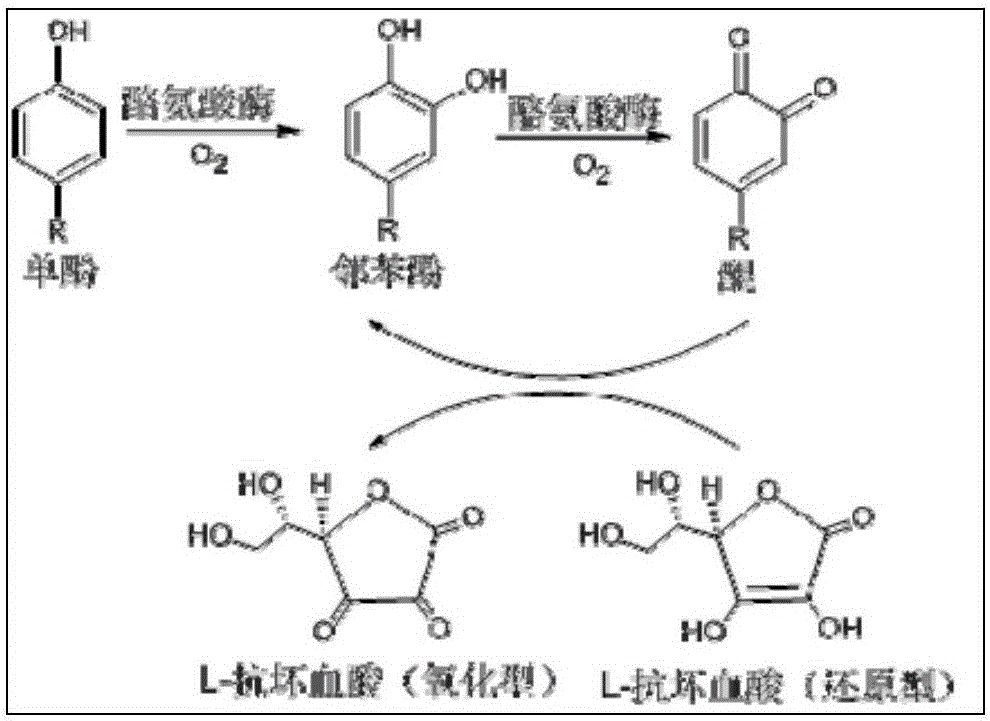
[0046] Embodiment 1 Preparation of carrier-free immobilized tyrosinase
[0047] Wash and dry the fresh white Agaricus bisporus bought in the market and mash it with a homogenizer according to the ratio of mushroom: phosphate buffer (50mM, pH6.0)=1:2 (mass-volume ratio, g / ml), The residue was removed by filtration, and the enzyme solution was divided and stored in a -20°C refrigerator. Thaw the frozen enzyme solution at room temperature, add ammonium sulfate to 50% saturation, continue shaking, after ammonium sulfate is completely dissolved, add 25% glutaraldehyde (wt.%) to make the glutaraldehyde in the enzyme solution The amount reached 0.25% (wt.%), and the stirring was continued at 4°C for 16h. Centrifuge the resulting turbid solution (10min, 8000rmp, 4°C), remove the supernatant, add phosphate buffer (pH6.0, 50mM) to wash the precipitate, then centrifuge (10min, 8000rmp, 4°C) and repeat the washing 4 times until the supernatant is completely Until there is no enzyme acti...
Embodiment 2
[0048] Example 2 HPLC Determination of Picetanol and 3'-Hydroxy Pterostilbene Concentrations
[0049] High-performance liquid chromatography adopts reverse C18 column (Japan GL Sciences, Inc company, Inertsil ODS-SP, 4.6 * 150mm, 5 μ m) to measure the content of piceatanol and 3'-hydroxy pterostilbene, and the liquid phase condition is: mobile phase A is: 0.5% acetic acid / acetonitrile=95 / 5 (v / v), mobile phase B is: acetonitrile / 0.5% acetic acid=95 / 5 (v / v), A / B=75 / 25 (v / v) ; Injection volume: 10 μL; total flow rate is 1ml / min; detection wavelength of piceatanol is 320nm, detection wavelength of 3'-hydroxy pterostilbene is 306nm; column temperature is 29°C.
[0050] Precisely prepare standard solutions of piceatanol and 3'-hydroxy pterostilbene with acetonitrile, each series is repeated 3 times, and the peak area is obtained by HPLC detection and the average value is obtained. The concentration (X, mM) is the abscissa, and the peak area (Y) is the ordinate to draw the standard ...
Embodiment 3
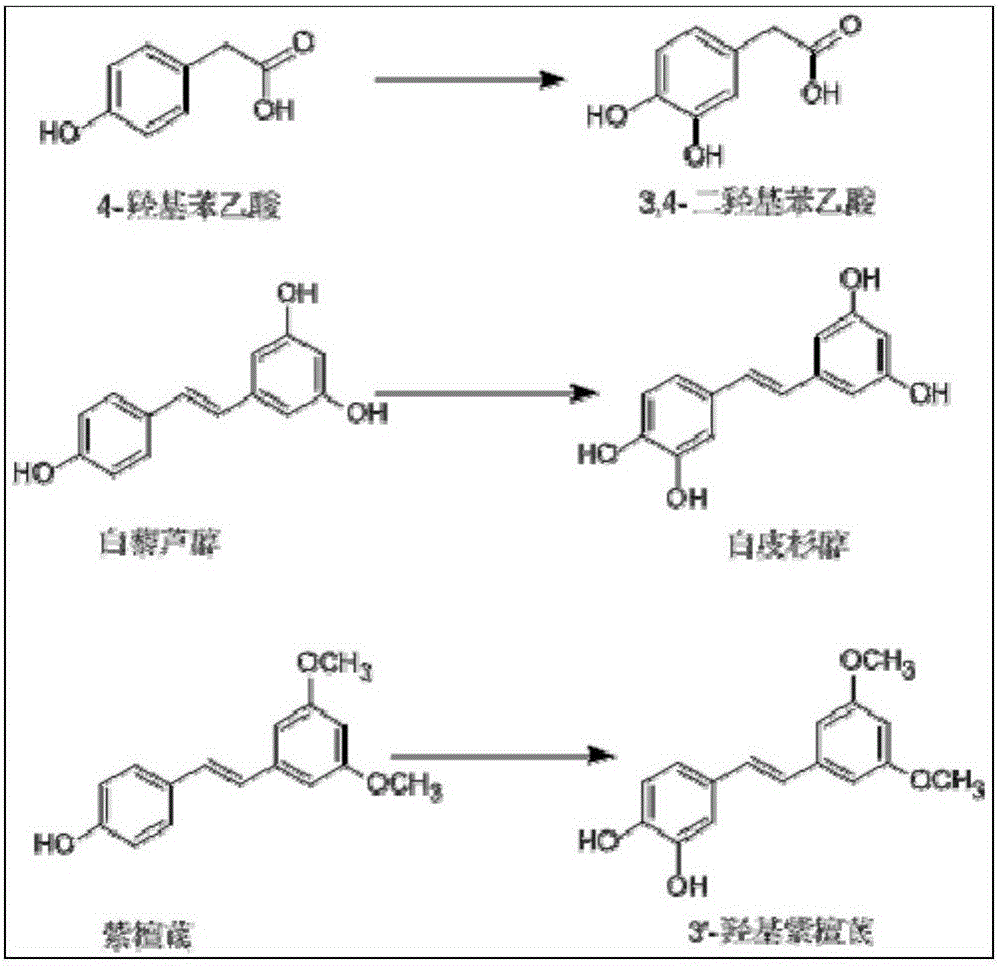
[0051] Example 3 Preparation of 3'-hydroxy pterostylbene by enzymatic method
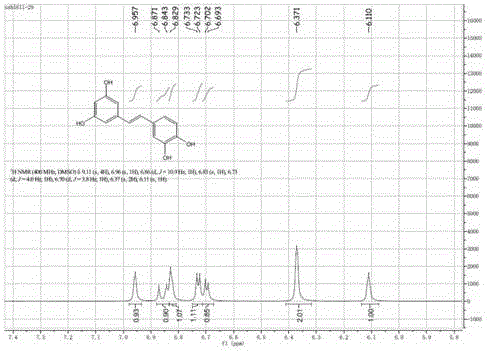
[0052] Accurately weigh 29% (wt.%) of carrier-free immobilized tyrosinase, the amount of pterostilbene added is 30% (wt.%), and the amount of L-ascorbic acid is 41% (wt.%). 25% (v / v%) DMSO was used to aid dissolution, and the reaction was carried out at 20° C., in a constant temperature shaking incubator at 220 rpm. After reacting for 1, 2, 3, 4, 5, 6, and 7 hours, centrifuge at 6000 rpm for 2 minutes, take 20 μl of supernatant, and put the rest of the reaction solution back into the constant temperature shaking incubator to continue the reaction. Figure 4 shown. The supernatant was diluted 20 times (diluted to 400 μl), vortexed and shaken to mix evenly, filtered, and the content of the substrate and product was detected by HPLC, such as Figure 5 As shown, the yield of 3'-hydroxy pterostylbene reached the maximum when the reaction was carried out to 5h, which was 61.8%. react as figure 1 and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com