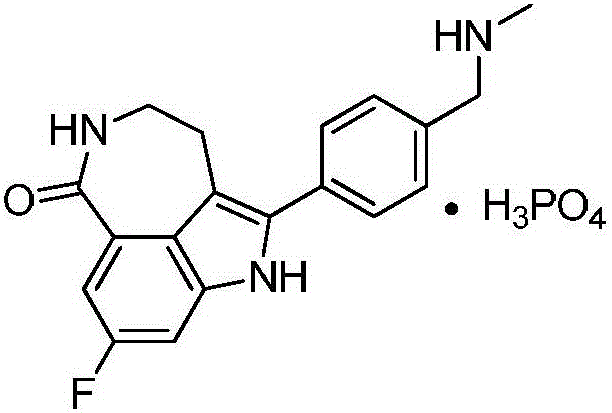
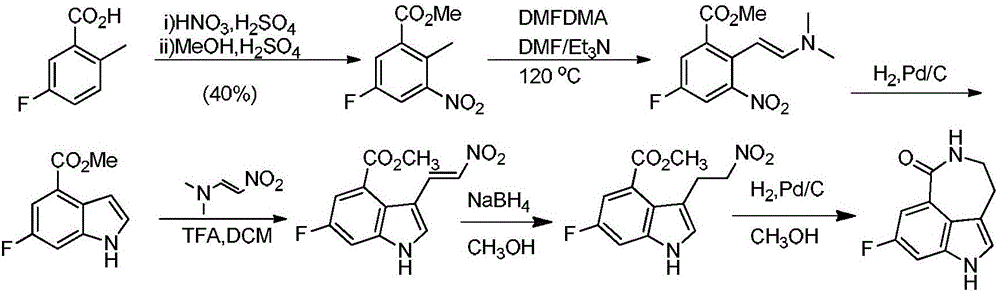
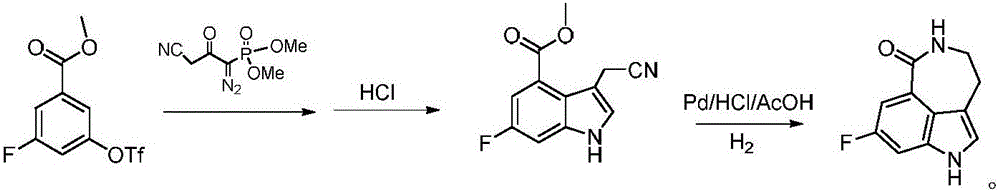
Preparation method of intermediate of drug Rucaparib treating ovarian cancer
An intermediate and ovarian cancer technology, applied in the field of preparation of ovarian cancer drug Rucaparib intermediates, can solve the problems of long reaction time, complicated process, harsh conditions, etc., and achieve the effect of short reaction time, high reaction efficiency and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0032] Preparation of Dimethyl (1-diazo-2-oxo-3-cyanopropyl)phosphonate
[0033] Under ice-cooling, drop 220ml LiHMDS (1mol / L in THF) into 13.6g (110mmol) of dimethyl methylphosphonate for mixing, then add 9.9g (100mmol) of methyl 2-cyanoacetate to the above mixture , kept stirring in an ice bath for 2 hours, and monitored the completion of the reaction. Add 100ml of saturated ammonium chloride to quench the reaction, add ethyl acetate to extract three times (80mlx3), combine the organic phases, wash with saturated brine, dry over anhydrous magnesium sulfate, and concentrate to obtain (2-oxo-3-cyanopropyl) The crude dimethyl phosphonate was used in the next step without further purification.
[0034] The obtained (2-oxo-3-cyanopropyl) dimethyl phosphonate crude product, p-toluenesulfonyl azide 23.7g (TsN 3 120mmol) was dissolved in acetonitrile, then 20.7g (150mmol) of potassium carbonate was added, stirred at room temperature for 8 hours, filtered, the filtrate was concentr...
preparation example 2
[0037] Preparation of Dimethyl (1-diazo-2-oxo-3-cyanopropyl)phosphonate
[0038] Under ice-cooling, drop 200ml LiHMDS (1mol / L in THF) into dimethyl methylphosphonate 14.8 (120mmol) for mixing, then add 9.9g (100mmol) of methyl 2-cyanoacetate to the above mixture, The reaction was kept stirring in an ice bath for 2 hours, and the completion of the reaction was monitored. Add 100ml of saturated ammonium chloride to quench the reaction, add ethyl acetate to extract three times (80mlx3), combine the organic phases, wash with saturated brine, dry over anhydrous magnesium sulfate, and concentrate to obtain (2-oxo-3-cyanopropyl) The crude dimethyl phosphonate was used in the next step without further purification.
[0039] The obtained (2-oxo-3-cyanopropyl) dimethyl phosphonate crude product, p-toluenesulfonyl azide 21.7g (TsN 3 110mmol) was dissolved in acetonitrile, then 22.1g (160mmol) of potassium carbonate was added, the reaction was stirred at room temperature for 7 hours, fi...
Embodiment 1
[0041] Preparation of 3-cyanoethyl-6-fluoro-1H-indole-4-carboxylic acid methyl ester
[0042] 1) Under the protection of nitrogen, first mix 33.2g (110mmol) of methyl 3-fluoro-5-trifluoromethanesulfonylbenzoate and 130.3g (400mmol) of cesium carbonate in 180ml tetrahydrofuran for 5-10min, then add ( 21.7 g (100 mmol) of dimethyl 1-diazo-2-oxo-3-cyanopropyl)phosphonate was stirred and reacted at room temperature for 1.5 hours. After the stirring reaction was completed, the reaction solution was concentrated under reduced pressure and washed with water to obtain a mixture M;
[0043] 2) Mix the mixture M obtained in step 1) with 50ml of hydrochloric acid (6mol / L,) in 150ml of tetrahydrofuran, heat up to 55°C and stir for reaction. After the reaction is completed for 2 hours, adjust the pH to 7-8 with sodium bicarbonate, ethyl acetate Extraction and concentration under reduced pressure gave 20.8 g of methyl 3-cyanoethyl-6-fluoro-1H-indole-4-carboxylate, with a yield of 89.6%. HR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com