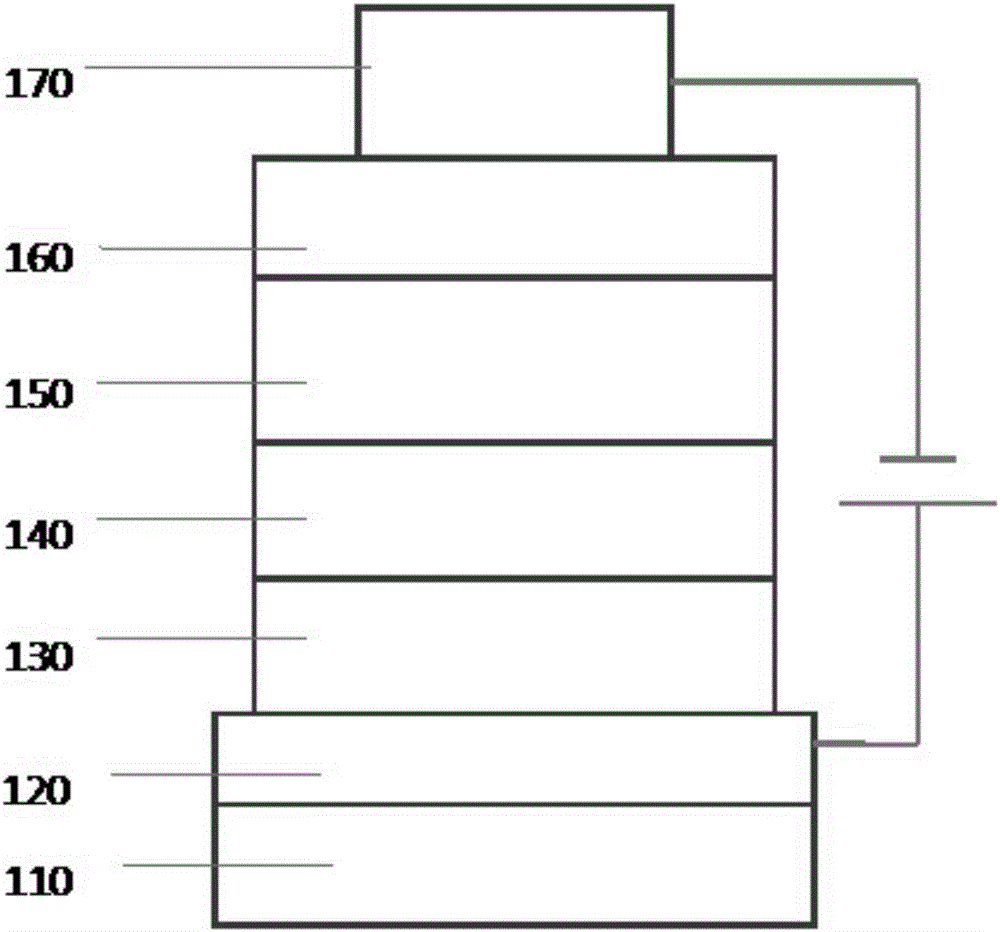
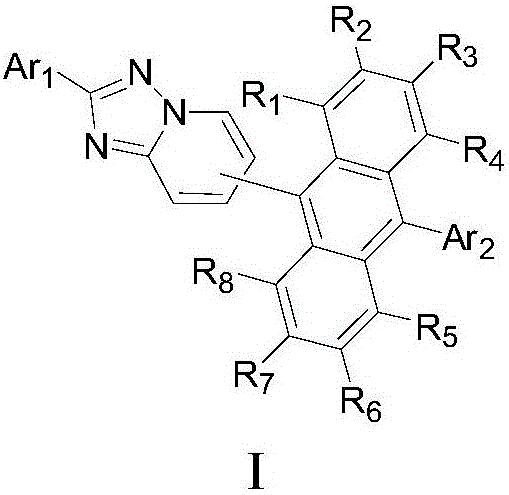
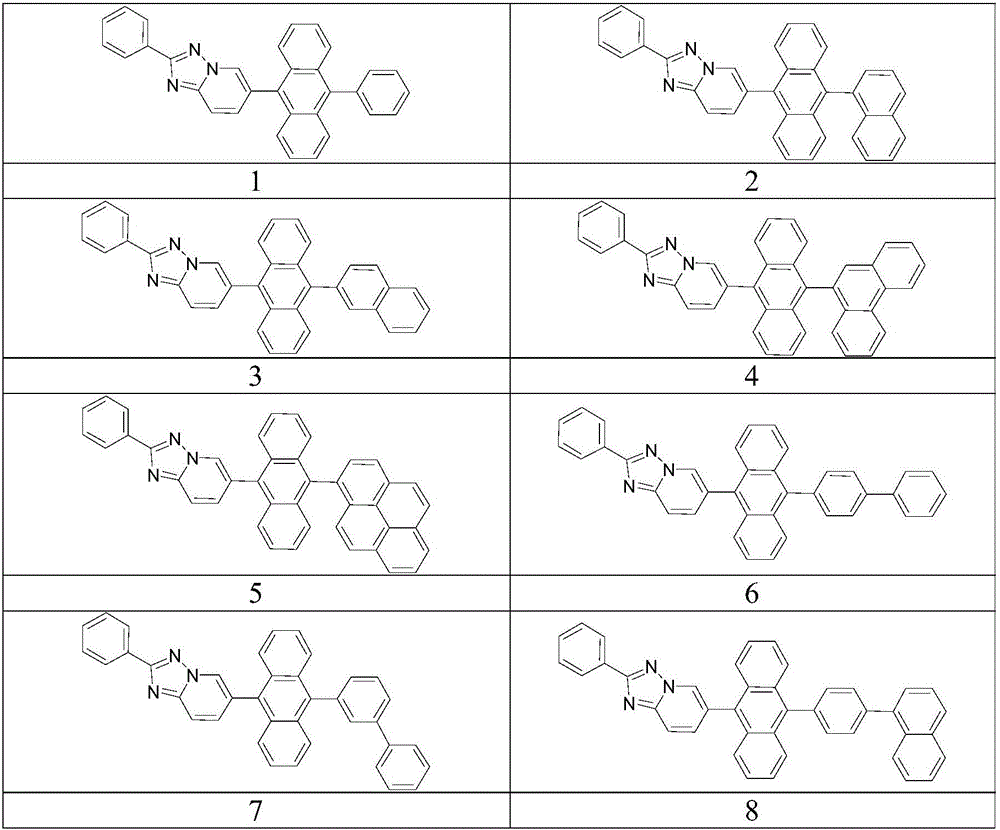
Anthracene-based organic electroluminescent electron transport compound and light-emitting apparatus thereof
An electron transport and compound technology, applied in organic chemistry, electro-solid devices, electrical components, etc., can solve the problems of easy crystallization, low electron mobility and low electron mobility of organic materials, and achieve good electroluminescence efficiency, The effect of high luminous purity and high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Synthesis of Compound 2
[0044]
[0045] Synthesis of Intermediate 2-1
[0046] In the reaction flask, benzonitrile (21g, 0.2mol), 5-bromo-2-aminopyridine (34g, 0.2mol), cuprous bromide (1g), 1,10phenanthroline (1g), zinc iodide ( 5g), ortho-dichlorobenzene (300ml), reacted at 130°C for 24h, cooled to room temperature and filtered to remove inorganic salts, and the filtrate was concentrated and separated by column chromatography to obtain 28g of white solid with a yield of 51%.
[0047] Synthesis of Compound 2
[0048] In the reaction flask, add intermediate 2-1 (1.5g, 5.5mmol), 9-(1-naphthalene)-10-anthracene boronic acid (1.9g, 5.5mmol), potassium carbonate (1.5g, 11mmol), tetrahydrofuran ( 30ml), water (10ml), tetrakistriphenylphosphine palladium (0.1g) was heated to reflux under nitrogen protection for 12 hours, cooled, removed the solvent, extracted with dichloromethane, dried and concentrated, and the crude product was purified by column chromatography to ob...
Embodiment 2
[0050] Synthesis of Compound 3
[0051]
[0052] The synthesis method was the same as that of compound 2, except that 9-(1-naphthalene)-10-anthraceneboronic acid was replaced by 9-(2-naphthalene)-10-anthraceneboronic acid, and the yield was 73%. Elemental analysis: C, 86.60; H, 4.96; N, 8.44.
Embodiment 3
[0054] Synthesis of Compound 8
[0055]
[0056] The synthesis method was the same as that of compound 2, except that 9-(4-(1-naphthalene)-phenyl)-10-anthraceneboronic acid was used instead of 9-(1-naphthalene)-10-anthraceneboronic acid, and the yield was 57%. Elemental analysis: C, 87.92; H, 4.75; N, 7.23.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com