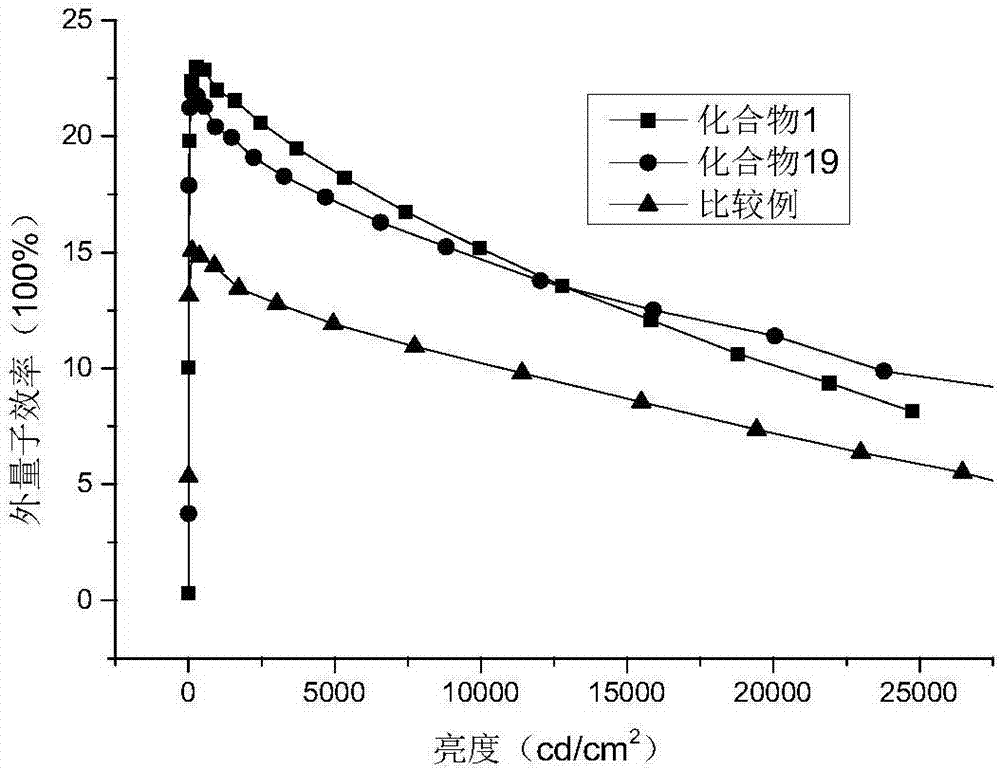
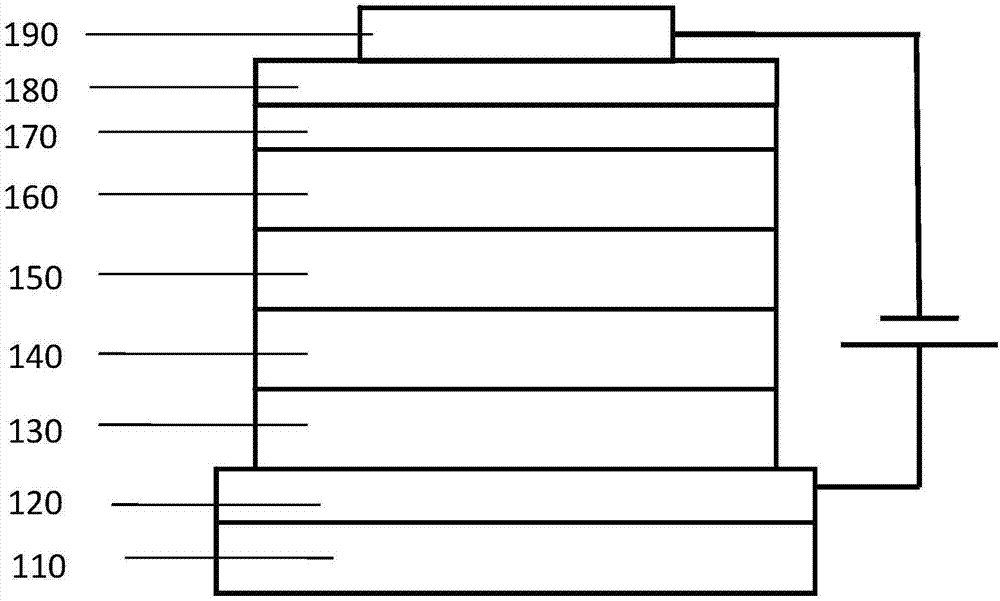
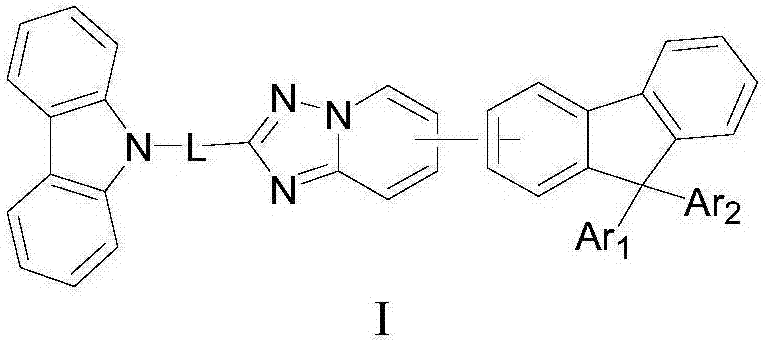
Heterocyclic organic electroluminescence compound and organic electroluminescence device thereof
A compound and luminescence technology, applied in organic chemistry, electrical solid devices, electrical components, etc., can solve problems such as charge imbalance in the light-emitting layer, lower device efficiency, and difficult flow of electrons, and achieve high luminous purity and good thermal stability , the effect of high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Synthesis of compound 1
[0047]
[0048] Synthesis of Intermediate 1-1
[0049] Add p-bromobenzonitrile (5g, 27.5mmol), carbazole (4.2g, 25mmol), sodium tert-butoxide (4.8g, 50mmol), palladium acetate, x-phos, 50ml toluene in a single-necked flask, Under heating and reflux for 24h, cooling. Remove toluene by rotary evaporation, add 50ml of dichloromethane to dissolve, and wash with water. The organic phase was spin-dried, added ethanol for recrystallization, and the product was filtered out with suction. 3.9 g of a white solid was obtained with a yield of 60%.
[0050] Synthesis of intermediate 1-2
[0051] Add intermediate 1-1 (1g, 3.7mmol), 5-bromo-2-aminopyridine (0.78g, 4.5mmol), cuprous bromide (0.18mmol, 27mg), o-phenanthroline (0.18 mmol, 33mg), zinc iodide (0.37mmol, 118mg), o-dichlorobenzene (5mL), reacted at 130°C for 24 hours under nitrogen protection, diluted with ethyl acetate after cooling to room temperature, and filtered to remove inorganic salt...
Embodiment 2
[0054] Synthesis of Compound 8
[0055]
[0056] The synthesis method was the same as that of compound 1, except that 9,9'-spirobis[9H-fluorene]-2-boronic acid was replaced by 9,9-diphenylfluorene-4-boronic acid, and the yield was 74%.
Embodiment 3
[0058] Synthesis of Compound 13
[0059]
[0060] Synthesis of Intermediate 13-1
[0061] The synthesis method is the same as that of intermediate 1-2, except that the raw materials used are 9-(3-cyanobenzene)-carbazole and 2-amino-4-bromopyridine, and the yield is 44%.
[0062] Synthesis of Compound 13
[0063] The synthesis method is the same as that of compound 1, and the raw materials used are intermediate 13-1 and 9,9-diphenylfluorene-2-boronic acid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com