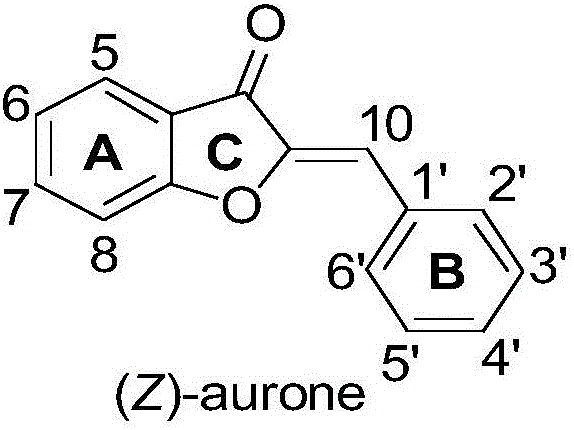
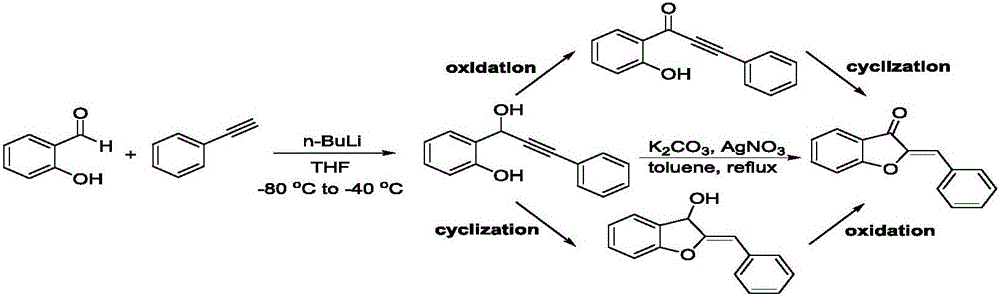
Synthesis method of aurone compound
A synthesis method and compound technology, applied in biocides, organic chemistry, chemicals for biological control, etc., can solve the problems of many reaction steps and complicated operation, and achieve simple reaction steps, good stereoselectivity, and yield high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Step 1: Add 16.4g of phenylacetylene into a double-necked round-bottom flask, add a magnetic rotor, quickly evacuate, replace the air with an inert gas, repeat twice, seal it for later use, and add anhydrous THF to it under the protection of an inert gas , Stir evenly at room temperature, put it in a -40°C ethanol bath and stir for later use, keep it at -40°C, slowly add 9.6g of n-butyllithium to it, react for 30 minutes, slowly add 12.2g of 2-hydroxybenzaldehyde for 3 hours , add saturated ammonium chloride solution to quench neutralization, remove THF by rotary evaporation, add ethyl acetate for extraction, collect the organic phase, wash the organic phase with saturated brine, add sodium sulfate to remove water and filter, collect the filtrate, and distill under reduced pressure After removing ethyl acetate, pass through a silica gel column with chloroform and petroleum ether at a volume ratio of 1:1 as the eluent to obtain 17.5 g of an intermediate product with a yie...
Embodiment 2
[0031] Step 1: Add 16.4g of phenylacetylene into a double-necked round-bottom flask, add a magnetic rotor, quickly evacuate, replace the air with an inert gas, repeat twice, seal it for later use, and add anhydrous THF to it under the protection of an inert gas , Stir evenly at room temperature, put it in an ethanol bath at minus 40°C and stir for later use, keep it at -40°C, slowly add 9.6g of n-butyllithium to it, react for 30 minutes, and slowly add 23.4g of 3,5-di-n-butyl 2-Hydroxybenzaldehyde was reacted for 2 hours, and saturated ammonium chloride solution was added to quench the neutralization. After THF was removed by rotary evaporation, ethyl acetate was added for extraction, and the organic phase was collected. The organic phase was washed with saturated brine, and sodium sulfate was added to remove water. Filtrate, collect the filtrate, remove ethyl acetate by distillation under reduced pressure, pass through a silica gel column, use chloroform and petroleum ether at...
Embodiment 3
[0037] Step 1: Add 21.8g of p-chlorophenylacetylene into a double-necked round-bottomed flask, add a magnetic rotor, quickly evacuate, replace the air with an inert gas, repeat twice, seal it for later use, and add inert gas to it under the protection of an inert gas. Water THF, stirred evenly at room temperature, then put into -40°C ethanol bath and stirred for later use, kept at -40°C, slowly added 9.6g of n-butyl lithium dropwise to it, reacted for 30 minutes, slowly added dropwise 23.4g of 2-hydroxybenzene Formaldehyde was reacted for 0.5 hours, adding saturated ammonium chloride solution to quench the neutralization, rotary evaporation to remove THF, adding ethyl acetate for extraction, collecting the organic phase, washing the organic phase with saturated brine, adding sodium sulfate to remove water and filtering, collecting the filtrate , after distilling off ethyl acetate under reduced pressure, pass through a silica gel column with chloroform and petroleum ether at a v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com