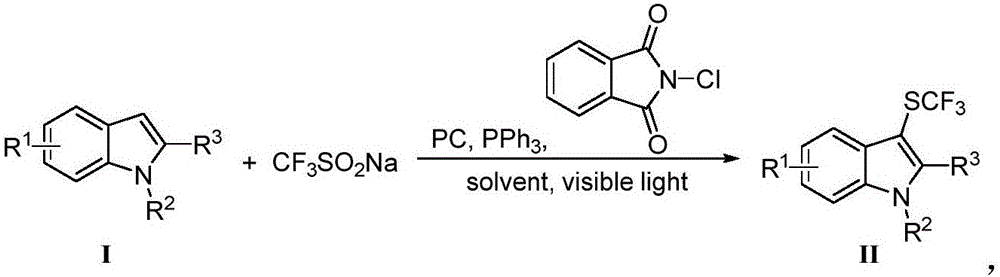
Method for preparing trifluoro-methylmercapto-substituted indole compound
A technology of trifluoromethylthio and trifluoromethyl, which is applied in the field of preparation of trifluoromethylthio substituted indole compounds, can solve problems such as complex preparation process, and achieve stable chemical properties, low toxicity and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
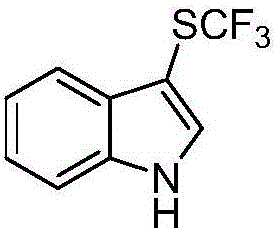
[0013] Embodiment 1: Preparation of 3-trifluoromethylthioindole
[0014]
[0015] Eosin Y (0.01 mmol), sodium trifluoromethanesulfinate (0.3 mmol), triphenylphosphine (0.6 mmol), N-chlorophthalimide (0.3 mmol) were added to a 10 mL Schlenk tube , flushed into dry nitrogen after vacuuming (this process was repeated three times), indole (0.2 mmol) was dissolved in the ultra-dry solvent acetonitrile and the resulting solution was injected into the Schlenk tube through a syringe. The reaction was illuminated and stirred under a white LED light for 6 hours at room temperature. After the reaction was completed, the solvent was removed by rotary evaporation, and the residue was purified by silica gel column chromatography to obtain 37 mg of the product with a yield of 85%.
[0016] 1 H NMR (500MHz, CDCl 3 )δ8.45(br,1H),7.92–7.79(m,1H),7.51(d,J=2.8Hz,1H),7.45–7.39(m,1H),7.37–7.28(m,2H). 13 C NMR (125MHz, CDCl 3 )δ136.2, 133.0, 129.6 (q, J=309.7Hz), 129.6, 123.6, 121.8, 119.5, ...
Embodiment 2
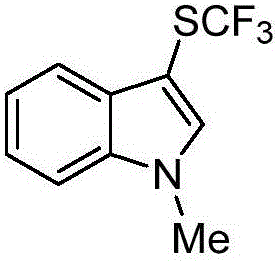
[0017] Embodiment 2: Preparation of 1-methyl-3-trifluoromethylthioindole
[0018]
[0019] Eosin Y (0.01 mmol), sodium trifluoromethanesulfinate (0.3 mmol), triphenylphosphine (0.6 mmol), N-chlorophthalimide (0.3 mmol) were added to a 10 mL Schlenk tube , flushed into dry nitrogen after vacuuming (this process was repeated three times), 1-methylindole (0.2 mmol) was dissolved in the ultra-dry solvent acetonitrile and the resulting solution was injected into the Schlenk tube through a syringe. The reaction was illuminated and stirred under a white LED light for 6 hours at room temperature. After the reaction, the solvent was removed by rotary evaporation, and the residue was purified by silica gel column chromatography to obtain 43 mg of the product with a yield of 93%.
[0020] 1 H NMR (500MHz, CDCl 3 )δ7.85(d,J=7.6Hz,1H),7.40–7.31(m,4H),3.80(s,3H). 13 C NMR (125MHz, CDCl 3 )δ137.4, 137.1, 129.6 (q, J=310.4Hz), 130.4, 123.1, 121.4, 119.5, 110.0, 93.2, 33.3. 19 F NMR (...
Embodiment 3
[0021] Embodiment 3: Preparation of 1-benzyl-3-trifluoromethylthioindole
[0022]
[0023] Eosin Y (0.01 mmol), sodium trifluoromethanesulfinate (0.3 mmol), triphenylphosphine (0.6 mmol), N-chlorophthalimide (0.3 mmol) were added to a 10 mL Schlenk tube , flushed into dry nitrogen after vacuuming (this process was repeated three times), 1-benzyl indole (0.2 mmol) was dissolved in the ultra-dry solvent acetonitrile and the resulting solution was injected into the Schlenk tube through a syringe. The reaction was illuminated and stirred under a white LED light for 6 hours at room temperature. After the reaction was completed, the solvent was removed by rotary evaporation, and the residue was purified by silica gel column chromatography to obtain 57 mg of the product with a yield of 93%.
[0024] 1 H NMR (500MHz, CDCl 3 )δ7.85(d, J=7.0Hz, 1H), 7.47(s, 1H), 7.36–7.28(m, 6H), 7.16(d, J=6.5Hz, 2H), 5.34(s, 2H). 13 C NMR (125MHz, CDCl 3 )δ137.0, 136.5, 136.2, 130.6, 129.4 (q, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com