Method for detecting newly increased detection components in traditional Chinese medicinal Tang Herb for treating AIDS
A determination method and technology of Tangcao Tablets are applied in the field of determination of newly detected components in Chinese medicine Tangcao Tablets, which can solve the problems of cumbersome operation, uncontrollable other components, and unfavorable detection of large batches of samples, so as to improve product quality, and the determination method is stable and reliable. , the effect of large application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
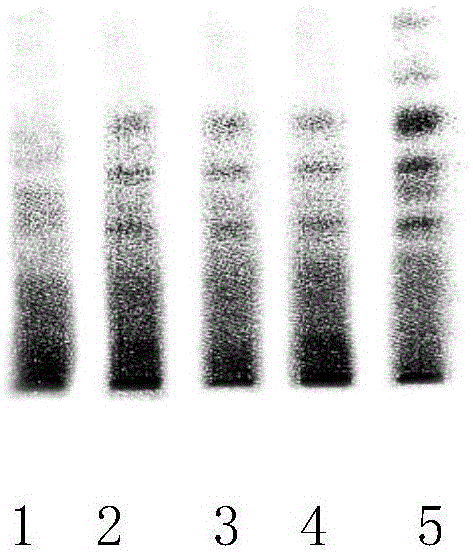
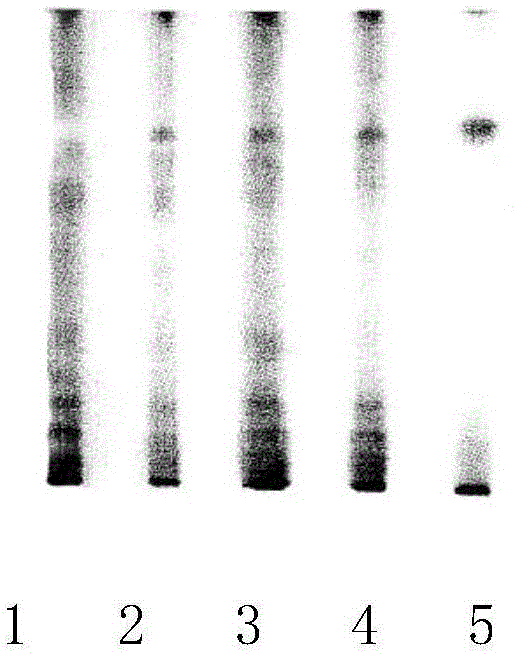
[0038] Identification of Hedyotis diffusa in Tangcao Tablets: Take Tangcao Tablet Sample 1, Sample 2, and Sample 3 (all produced by Shanghai Baisuihang Pharmaceutical Co., Ltd., commercially available products) each 10 pieces (each piece weighs 0.4g) , grind finely, add 50ml of water, sonicate for 30min, centrifuge, take the supernatant, add 50ml of chloroform (all reagents used are commercially available), extract once, collect the chloroform layer, evaporate to dryness, add 1.5ml of methanol to dissolve the residue As the test product. In addition, get Hedyotis diffusa reference medicinal material (purchased from China Institute for the Control of Biological Products) 0.5g, add water 20ml, heat and reflux for 1.5 hours, filter, add water to the filtrate to 50ml, from adding chloroform 50ml together for the test According to the thin-layer chromatography (Chinese Pharmacopoeia 2010 edition one VIB) test, draw each 10 μ l of the above-mentioned two kinds of solutions, point res...
Embodiment 2
[0040] Embodiment 2: the identification of Myrobalan in Tang Herbal Tablets: (Tang Herbal Tablets sample is the same as Example 1)
[0041] Get 1 g of myrobalan reference medicinal material (purchased from China Institute for the Control of Biological Products), add 20 ml of water, heat and reflux for 1.5 hours, filter, add water to the filtrate to 50 ml, and supply under the identification item of Hedyotis diffusa from adding 50 ml of chloroform. The test sample is operated to make a control medicinal material solution. According to thin-layer chromatography (Chinese Pharmacopoeia 2010 edition one VIB) test, draw each 10 μ l of the need testing solution under contrast medical material solution and embodiment 1 Hedyotis diffusa identification item, point respectively on the same silica gel G thin-layer plate, Use the lower layer of dichloromethane-glacial acetic acid-water (15:5:0.5) as a developing agent, develop, take out, dry in the air, spray with 10% sulfuric acid ethanol...
Embodiment 3
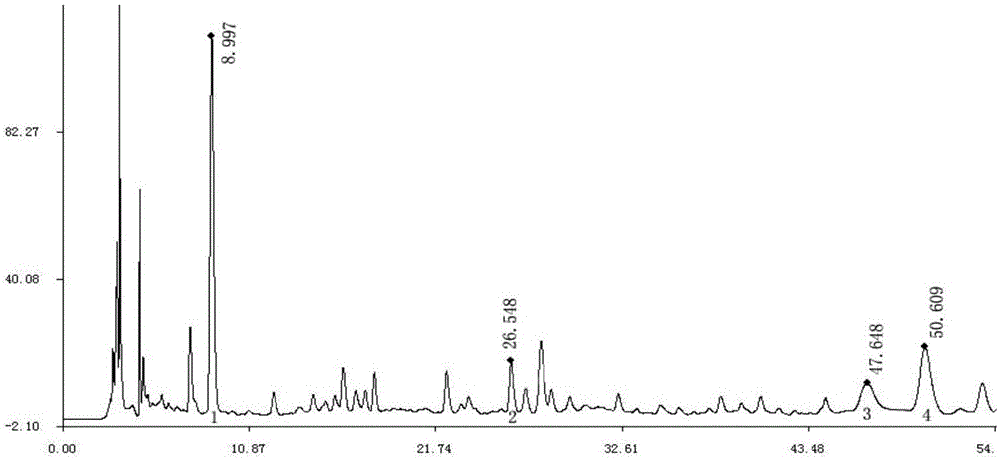
[0043] Example 3 Determination of gallic acid, chlorogenic acid, liquiritin, and berberine II in Tangcao tablets:
[0044] Take an appropriate amount of Tang Herbal Tablet samples (both are produced by Shanghai Baisuihang Pharmaceutical Co., Ltd., commercially available products), grind finely, take 1g, accurately weigh, put in a stoppered Erlenmeyer flask, accurately add 50% methanol 50ml, seal Stopper, weighed, ultrasonically treated for 30 minutes, taken out, placed at room temperature (25±2°C), weighed again, made up for the lost weight with methanol, shaken well, filtered, and it was the test solution, gallic acid , chlorogenic acid, liquiritin, and berberine II reference substance (purchased from China Institute for the Control of Biological Products):
[0045] Precisely weigh the appropriate amount of gallic acid reference substance, chlorogenic acid reference substance, liquiritin reference substance, and berberine Ⅱ reference substance, and add methanol to make each m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com