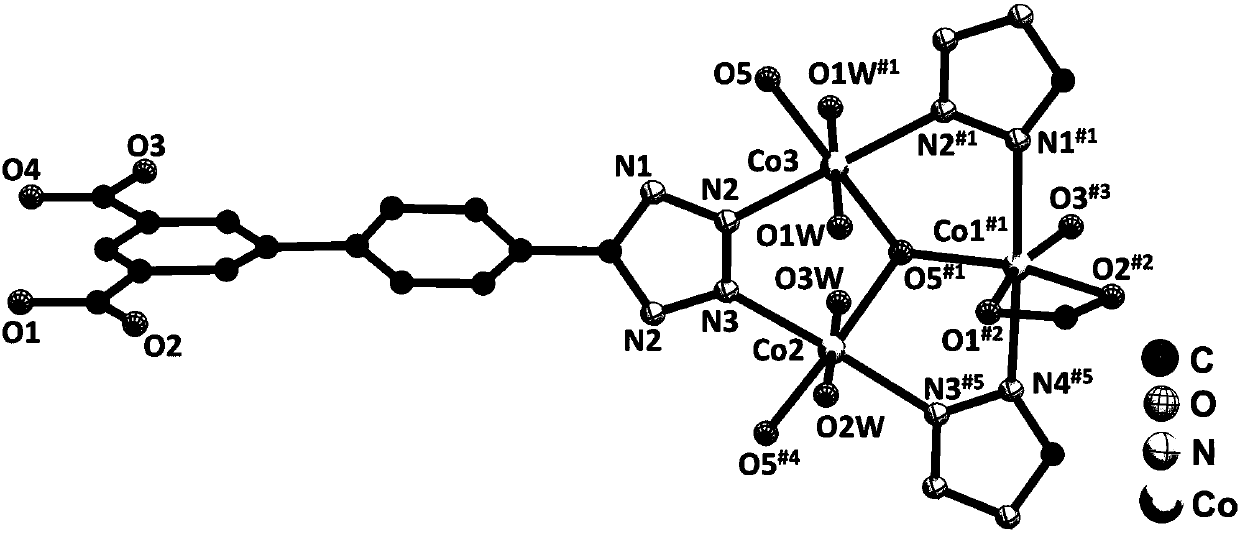
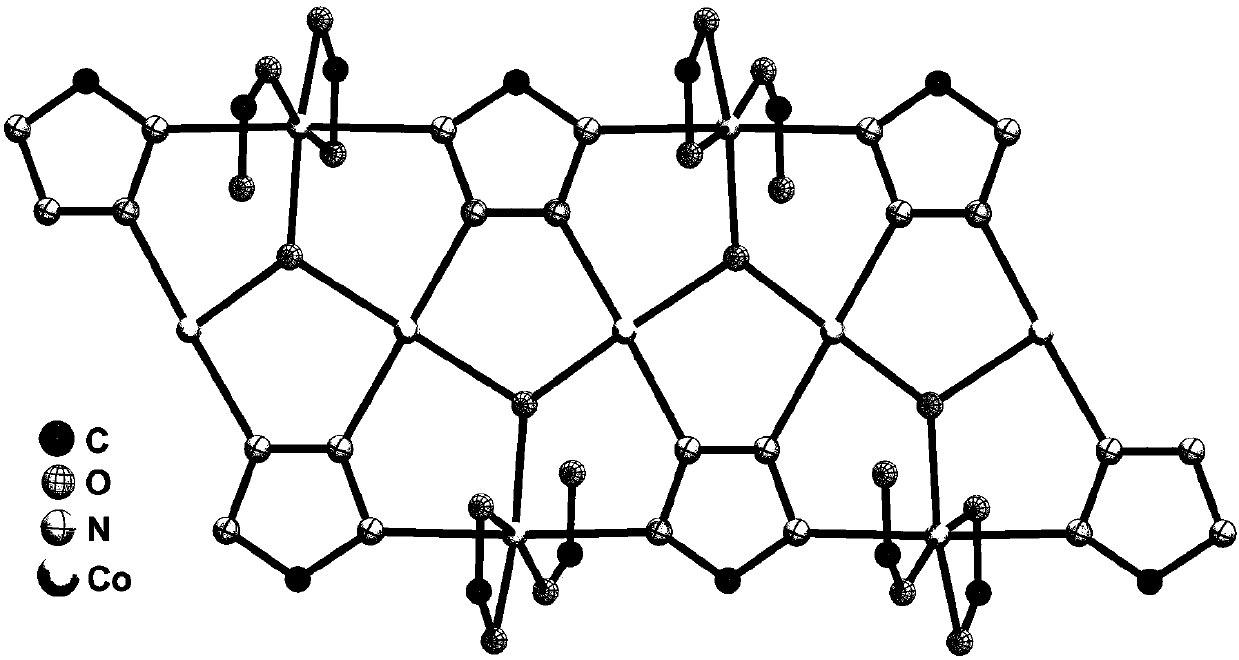
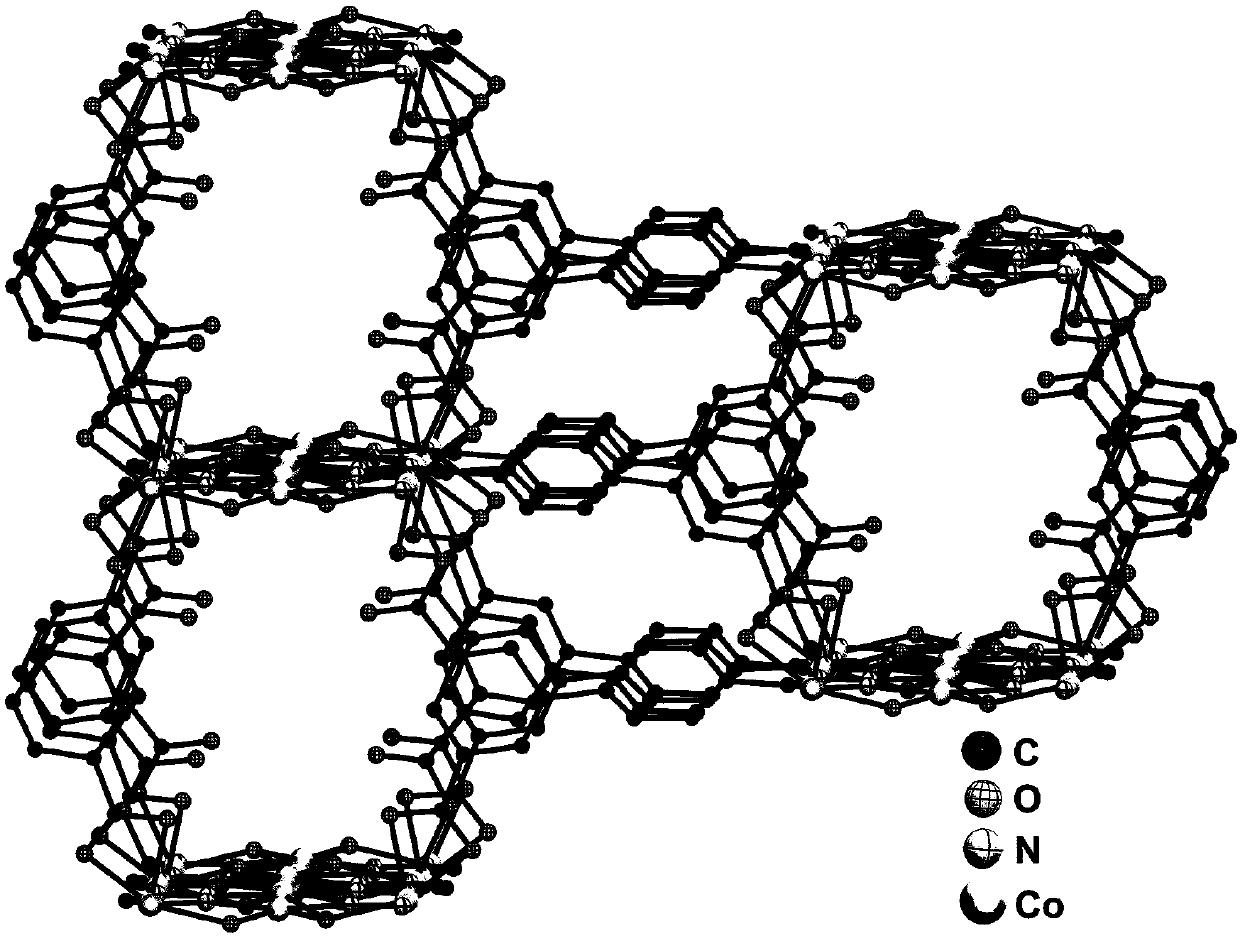
Preparation method and application of metal-organic framework material
A metal-organic framework and mass-part technology, applied in the field of inorganic and material chemistry science, can solve problems such as structural instability, and achieve the effects of low production cost, high safety factor, and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0045] A method for preparing a metal-organic framework material, comprising the steps of:
[0046] 1) Add 0.029 parts by mass of cobalt nitrate hexahydrate and 0.0155 parts by mass of 5-(4-(5-tetrazolyl)phenyl)isophthalic acid into a mixed solvent of N,N-dimethylformamide and water Mixed liquid is obtained after being stirred evenly in middle, wherein:
[0047] The mixed solvent includes 3.78 parts by mass of N,N-dimethylformamide and 3 parts by mass of water;
[0048] 2) Put the mixed liquid obtained in step 1) in a closed high-pressure reactor, heat it to 105°C and keep it warm for 72 hours, and then cool it down to room temperature at a rate of 0.1°C per minute to obtain a reaction product;
[0049] 3) filtering the reaction product obtained in step 2) to obtain red blocky crystals (i.e. the initial synthesis sample of the metal organic framework),
[0050] 4) The red blocky crystal obtained in step 3) was exchanged with methanol for 60 hours, and then heated in vacuum a...
specific Embodiment 2
[0070] A method for preparing a metal-organic framework material, comprising the steps of:
[0071] 1) Add 0.029 parts by mass of cobalt nitrate hexahydrate and 0.01 parts by mass of 5-(4-(5-tetrazolyl)phenyl)isophthalic acid into a mixed solvent of N,N-dimethylformamide and water Mixed liquid is obtained after being stirred evenly in middle, wherein:
[0072] The mixed solvent includes 3.5 parts by mass of N,N-dimethylformamide and 2.5 parts by mass of water;
[0073] 2) Place the mixed solution obtained in step 1) in a closed high-pressure reactor, heat it to 100°C and keep it warm for 80 hours, and then cool it down to room temperature at a rate of 0.15°C per minute to obtain a reaction product;
[0074] 3) filter the reaction product obtained in step 2) to obtain red blocky crystals,
[0075] 4) The red bulk crystals obtained in step 3) were exchanged with methanol for 70 hours, and then heated in vacuum at 220° C. for 5 hours to obtain metal organic framework materials....
specific Embodiment 3
[0086] A method for preparing a metal-organic framework material, comprising the steps of:
[0087] 1) Add 0.029 parts by mass of cobalt nitrate hexahydrate and 0.02 parts by mass of 5-(4-(5-tetrazolyl)phenyl)isophthalic acid to a mixed solvent of N,N-dimethylformamide and water Mixed liquid is obtained after being stirred evenly in middle, wherein:
[0088] The mixed solvent comprises 4.5 parts by mass of N,N-dimethylformamide and 3.5 parts by mass of water;
[0089] 2) Put the mixed liquid obtained in step 1) in a closed high-pressure reactor, heat it to 110°C and keep it warm for 65 hours, and then cool it down to room temperature at a rate of 0.2°C per minute to obtain a reaction product;
[0090] 3) filter the reaction product obtained in step 2) to obtain red blocky crystals,
[0091]4) The red blocky crystals obtained in step 3) were exchanged with methanol for 80 hours, and then heated in vacuum at 220° C. for 7 hours to obtain a metal organic framework material.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com