Medicine composition of lidocaine and application of medicine composition
A technology of lidocaine and composition, applied in the field of medicine, can solve problems such as troubles and blows of manufacturing enterprises
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0073] In the description of the present invention or the preparation process of each example, especially when the names of the components have been defined in the prescriptions of the examples, for the sake of simplification, the names of each component in the prescription can be simplified or omitted , for example, lidocaine hydrochloride 1 hydrate in the prescription can be referred to as lidocaine hydrochloride hydrate or lidocaine hydrochloride for short, sodium citrate 2 hydrate is referred to as sodium citrate or vice versa, and L- Menthol is referred to as menthol for short, and other components can be or analogously.
[0074] Concentration units used in the description of the present invention have molar concentration (M) or (mol / L) or equivalent concentration (N), or percentage concentration etc., time unit can use second (s), minute (min), hour (h) Etc., volume unit can use liter (l or L), milliliter (ml), microliter (μl), mass unit can use gram (g), milligram (mg) ...
Embodiment 1
[0079] The preparation of embodiment 1 compound lidocaine carbonate pharmaceutical composition injection (specification: 5ml / supports)
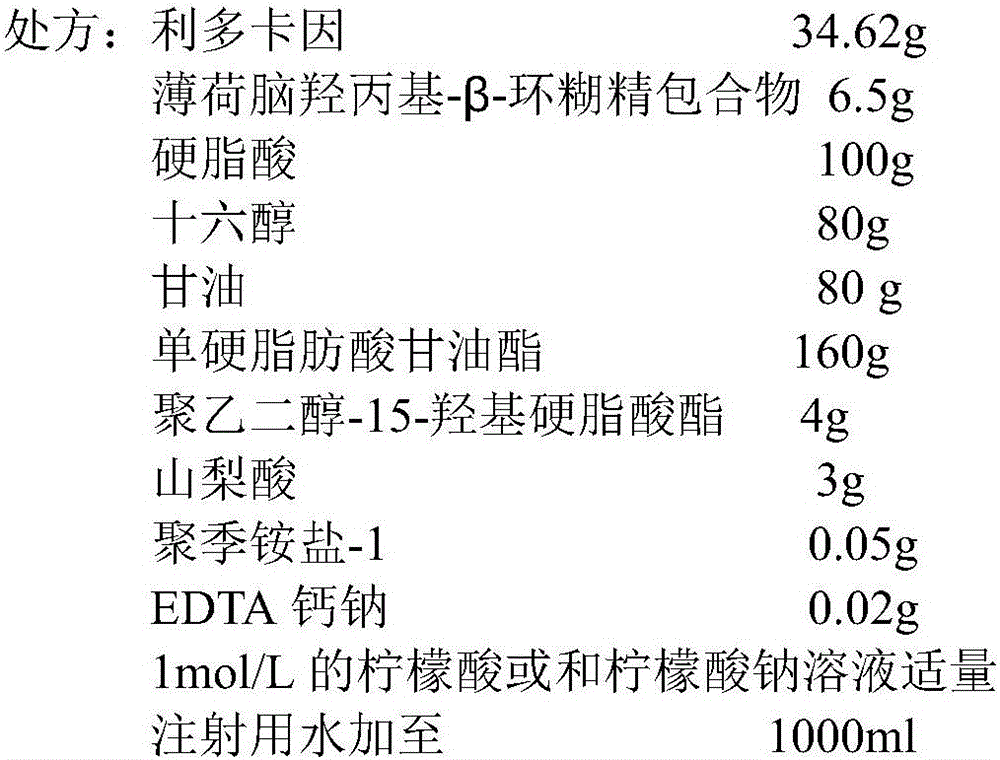
[0080] Prescription: Lidocaine hydrochloride monohydrate 34.61g (weight based on lidocaine net content), 2-hydroxypropyl-β-cyclodextrin inclusion compound of menthol 6.5g (based on menthol net content), 20g of aspartic acid, 250.0ml of glycerin, 10g of polyethylene glycol succinate, 0.05g of disodium edetate, an appropriate amount of 5% sodium bicarbonate solution, an appropriate amount of carbon dioxide gas, and water for injection to 5000ml;
[0081] Preparation process: ① Take prescription amounts of aspartic acid, glycerin, vitamin E polyethylene glycol succinate, disodium edetate and menthol hydroxypropyl-β-cyclodextrin inclusion compound, hydrochloric acid Dissolve lidocaine into a stainless steel bucket containing 4000ml of water for injection, stir and mix well; adjust the pH value of the solution to the range of 7.0 to 7.4 with an a...
Embodiment 2
[0083] The preparation of embodiment 2 compound lidocaine carbonate pharmaceutical composition injection preparation (specification: 10ml / supports)
[0084] Prescription: 69.23g of lidocaine hydrochloride monohydrate (based on the net content of lidocaine), 13.0g of 2-hydroxypropyl-β-cyclodextrin inclusion complex of L-menthol (based on the net content of menthol) , urea 20g, citric acid 15g, sodium citrate 15g, glycerin 350.0ml, vitamin E polyethylene glycol succinate 20g, edetate disodium 2 hydrate 1g, saturated sodium bicarbonate solution, carbon dioxide gas , add water for injection to 10L;
[0085] Preparation process: ① Take the prescribed amount of urea, citric acid, sodium citrate, glycerin, vitamin E polyethylene glycol succinate, disodium edetate and menthol hydroxypropyl-β-cyclodextrin respectively Dissolve the compound and lidocaine hydrochloride in a stainless steel bucket filled with 4000ml of water for injection, stir and mix well; adjust the pH value of the s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com