Sustained-release oral preparation of brivaracetam, and preparation method thereof
A sustained-release preparation, the technology of brivaracetam, applied in the field of pharmaceutical preparations, can solve the problems of large drug release, long dissolution time, and unfavorable patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 The raw material of brivaracetam was pulverized by airflow, so that the particle size distribution range of more than 85% was less than 30 microns, and more than 99% was less than 50 microns, and was set aside. Weigh 50 grams of processed brivaracetam raw materials, 200 grams of lactose monohydrate, 25 grams of microcrystalline cellulose, and 12 grams of crospovidone according to 1000 tablets and mix them uniformly. Add an appropriate amount of 5% hypromellose as Prepare wet granules with binder, dry them, add 2 grams of magnesium stearate and 2 grams of micropowder silica gel after granulation, mix evenly, press into tablets, the pressure is controlled at 4.5kg to 10kg, and each tablet is about 300mg.
[0023] Hardness: 6.4kg
[0024] Appearance: ++++
Embodiment 2
[0026] The raw material of buvaracetam is pulverized by air flow, so that the particle size distribution range of more than 85% is less than 30 microns, and more than 99% is less than 50 microns, and is ready for use. Weigh 50 grams of processed brivaracetam raw materials, 150 grams of starch, 180 grams of microcrystalline cellulose, and 18 grams of sodium carboxymethyl starch according to 1000 tablets, mix evenly, and add an appropriate amount of starch slurry with a concentration of 10% as a binder Prepare wet granules, dry them, add 4 grams of magnesium stearate and 2 grams of micro-powdered silica gel after sizing, mix evenly, press into tablets, the pressure is controlled at 4.5kg to 10kg, and each tablet is about 423mg.
[0027] Hardness: 4.8kg
[0028] Appearance: +++ Example 3 The raw material of brivaracetam is jet-milled, so that the particle size distribution range of more than 85% is less than 30 microns, and more than 99% is less than 50 microns, and is set aside....
Embodiment 4
[0032] The raw material of buvaracetam is pulverized by air flow, so that the particle size distribution range of more than 85% is less than 30 microns, and more than 99% is less than 50 microns, and is ready for use. Weigh 150 grams of processed buvaracetam raw materials, 100 grams of hydroxypropyl cellulose, 110 grams of microcrystalline cellulose, 10 grams of starch, and 10 grams of crospovidone according to 1000 pieces and mix them evenly, and the concentration of addition is 5%. (W / V) povidone ethanol solution, appropriate amount is used as a binder to prepare wet granules, dry, add 6 grams of magnesium stearate and 2 grams of micropowder silica gel after granulation, mix evenly, press into tablets, and the pressure is controlled at 4.5kg~ 10kg, about 400mg per tablet.
[0033] Hardness: 7.1kg
[0034] Appearance: +++
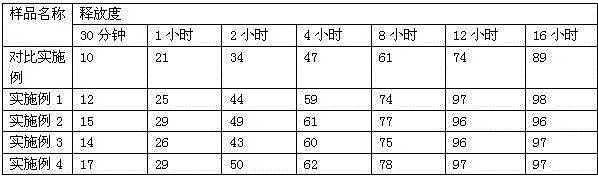
[0035] Dissolution release test results
[0036]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com