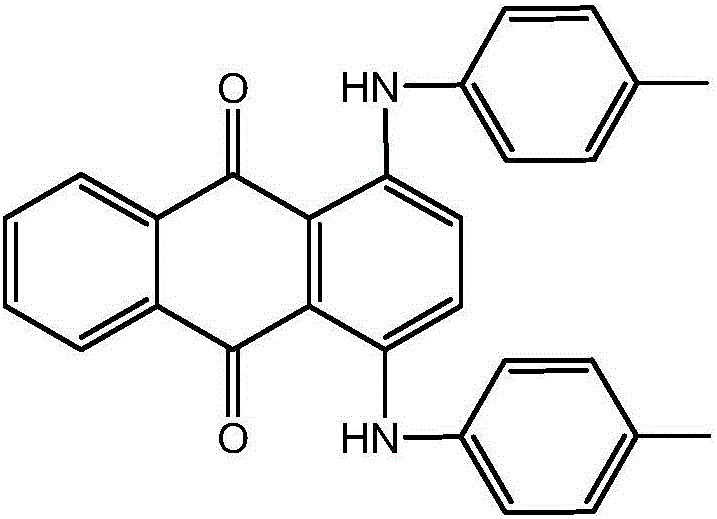
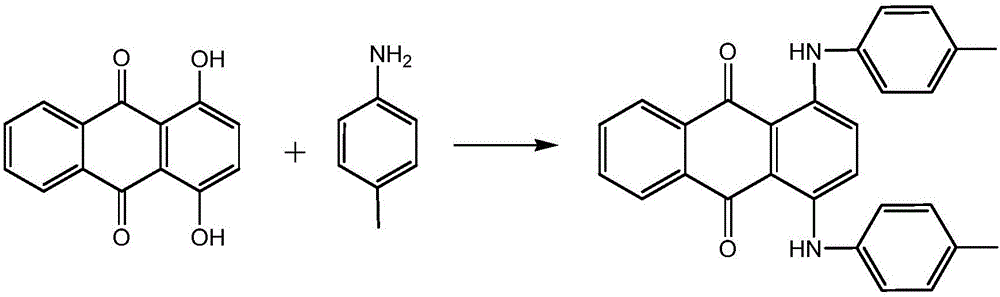
Synthesis method of solvent green 3
A synthesis method and solvent technology, applied in the synthesis field of solvent green 3, can solve the problems of low yield and high reaction temperature, and achieve the effects of improved initiation and catalysis, mild reaction conditions, and improved filterability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] In a 500mL four-necked flask with mechanical stirring and a thermometer, add 120g of ethanol, 60g of benzene, 2g of boric acid, 40g of 1,4-dihydroxyanthraquinone, 2g of sodium bisulfite, and 1,4-dihydroxyanthraquinone leuco After stirring evenly, 24 g of p-toluidine was added, and the temperature was raised to 64±2°C for dehydration reaction, and the reaction time was 12 hours. After the reaction, cool down to 20°C, filter, wash, and dry to obtain the target product 82.32g Solvent Green 3, with a purity of 98.5% and a yield of 97% (with 1,4-dihydroxyanthraquinone and 1,4-dihydroxyanthraquinone Anthraquinone leucosome total), shade (DC0.0, DH0.2, intensity 100.0), filter value 0.02.
Embodiment 2
[0028] In a 500mL four-neck flask equipped with mechanical stirring and a thermometer, add 160g of ethanol, 80g of benzene, 4g of boric acid, 40g of 1,4-dihydroxyanthraquinone, 4g of sodium bisulfite, and 1,4-dihydroxyanthraquinone leuco After stirring evenly, 23 g of p-toluidine was added, and the temperature was raised to 64±2°C for dehydration reaction, and the reaction time was 14 hours. After the reaction, cool down to 20°C, filter, wash, and dry to obtain 83.48g of the target product, Solvent Green 3, with a purity of 98.9% (based on the total of 1,4-dihydroxyanthraquinone and 1,4-dihydroxyanthraquinone leucosome ), the yield is 96.8%, shade (DC0.3, DH0.2, intensity 100.0), filter value 0.03.
Embodiment 3
[0030] In a 500mL four-necked flask with mechanical stirring and a thermometer, add 200g of ethanol, 100g of benzene, 6g of boric acid, 40g of 1,4-dihydroxyanthraquinone, 6g of sodium bisulfite, and 1,4-dihydroxyanthraquinone leuco After stirring evenly, 22 g of p-toluidine was added, and the temperature was raised to 64±2°C for dehydration reaction, and the reaction time was 16 hours. After the reaction, cool down to 20°C, filter, wash, and dry to obtain the target product 84.20g Solvent Green 3 with a purity of 99.5% (based on the total of 1,4-dihydroxyanthraquinone and 1,4-dihydroxyanthraquinone leucosome ), the yield is 96.3%, shade (DC0.6, DH0.4, intensity 100.0), filter value 0.01.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com