Crystal form of glucopyranose derivative
A technology of crystal forms and compounds, which is applied in the field of preparation of drugs as sodium-dependent glucose transporter inhibitors, can solve the problems that the crystallization behavior and results of organic pharmaceutical compounds cannot be predicted, and the structure and properties of crystal forms cannot be predicted.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0170] Example 1 Amorphous (1R,2S,3S,4R,5S)-5-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-1- Preparation of [(1R)-1-hydroxyethyl]-6,8-dioxobicyclo[3.2.1]octane-2,3,4-triol (compound I)
[0171]
[0172]
[0173] The synthetic route of compound (I) is shown above, wherein compound [(1S,2S,3S,4R,5S)-2,3,4-tribenzyloxy-5-[4-chloro-3-[(4 For the preparation method of -ethoxyphenyl)methyl]phenyl]-6,8-dioxobicyclo[3.2.1]octane-1-carbaldehyde (I-1), refer to the preparation method described in the patent WO2015043511, and in which The relevant contents of the above are incorporated into the present invention in their entirety.
[0174] Step 1 (1R)-1-[(1R,2S,3S,4R,5S)-2,3,4-tribenzyloxy-5-[4-chloro-3-[(4-ethoxyphenyl) Methyl]phenyl]-6,8-dioxabicyclo[3.2.1]octane-1-yl]ethanol (I-m)
[0175] At room temperature, the chiral ligand Cr-Salen (11.0 g, 17.4 mmol, 0.15 eq) was added to [(1S,2S,3S,4R,5S)-2,3,4-tribenzyloxy-5- [4-Chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-6,8-diox...
Embodiment 2
[0183] 1. Preparation of Form A
[0184] At room temperature, (1R,2S,3S,4R,5S)-5-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-1-[(1R)- 1-Hydroxyethyl]-6,8-dioxobicyclo[3.2.1]octane-2,3,4-triol (I) (1.00 g, 2.22 mmol) was dissolved in methanol (2 mL) while stirring While adding water (4 mL) to the solution, during the dropwise addition, the solution gradually changed from colorless and clear to white emulsion. After the dropwise addition, the resulting mixed system was stirred at room temperature for 2 hours, filtered with suction, and the filter cake was vacuum-dried at 50°C to constant weight (vacuum degree -0.098Mpa) to obtain a white solid. The solid was placed in an environment with a relative humidity higher than 60% to obtain crystal A (white solid, 0.96 g, yield 96%).
[0185] 2. Identification of Form A
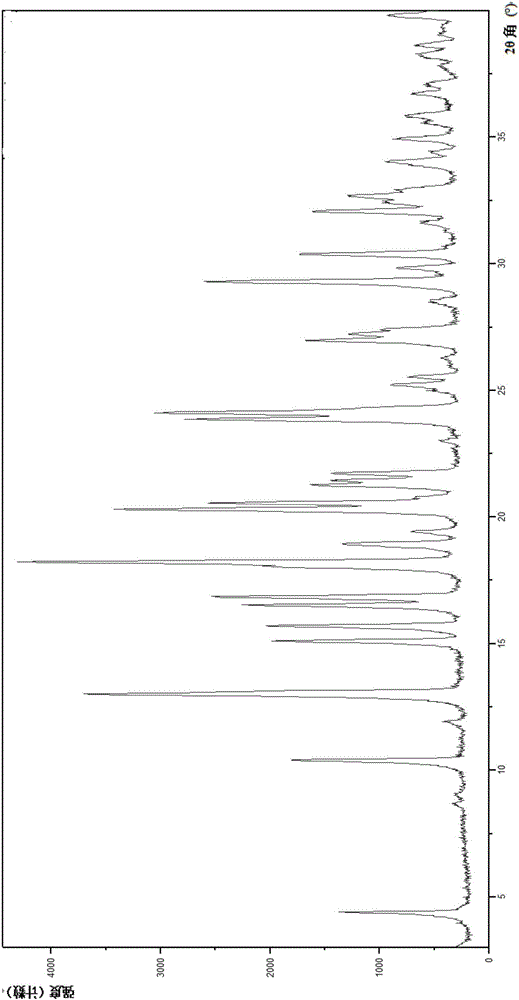
[0186] (1) X-ray powder diffraction (XRPD) analysis
[0187] The X-ray powder diffraction (XRPD) pattern of crystal form A is as follows figure 1 As shown, the sp...
Embodiment 3
[0198] 1. Preparation of Form B
[0199] At room temperature, (1R,2S,3S,4R,5S)-5-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-1-[(1R)- 1-Hydroxyethyl]-6,8-dioxobicyclo[3.2.1]octane-2,3,4-triol (I) (1.00 g, 2.22 mmol) was dissolved in methanol (2 mL) while stirring While adding water (4 mL) dropwise into the solution, the solution gradually changed from colorless and clear to white emulsion during the dropwise addition. After the dropwise addition, the resulting mixed system was stirred at room temperature for 2 hours, filtered with suction, and the filter cake was vacuum-dried at 50°C to constant weight (vacuum degree -0.098Mpa) to obtain a white solid. The solid was placed in an environment with a relative humidity lower than 40%, and crystal B (white solid, 0.96 g, yield 96%) was obtained.
[0200] 2. Identification of Form B
[0201] (1) X-ray powder diffraction (XRPD) analysis
[0202] The X-ray powder diffraction (XRPD) pattern of crystal form B is as follows Figure ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com