Preparation method of 2,2'-N,N-bis-pyrazolyl biphenyl polycarboxylic acid
A technology of bispyrazolyl biphenyl polycarboxylic acid and carboxyphenylboronic acid, which is applied in two fields, can solve the problems of few reports on pyrazolyl biphenyl carboxylic acid ligands, and achieve the effect of cheap range, economical and efficient reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
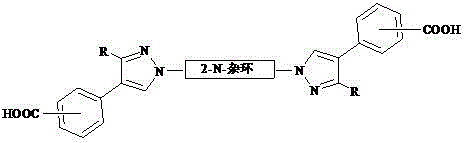
[0018] 2,2´-N, N-bispyrazolyl biphenyl polycarboxylic acid compound, the general formula is:
[0019]
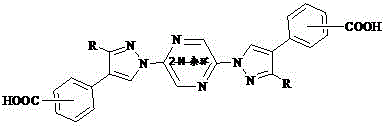
[0020] The specific structure can be:
[0021]
[0022]
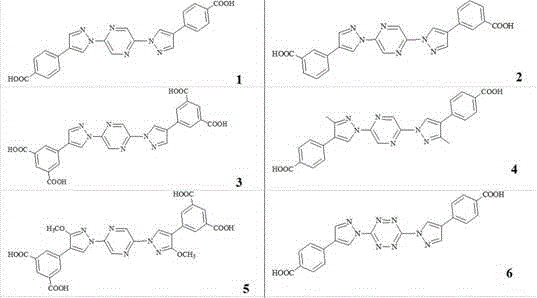
[0023]
[0024]
[0025]
Embodiment 2
[0027] Preparation of 2,2´-N, N-bispyrazolyl biphenyl polycarboxylic acid compound (1): under nitrogen protection, transfer to a 10 ml Schlek reaction tube (a glass commonly used in anhydrous and oxygen-free operation) instrument) by adding 1.0 mmol 2, 5-dibromopyrazine, 2.0 mmol 4-iodopyrazole, 0.01 mmol palladium acetate, 0.02 mmol di-tert-butyl (2',4',6'-triisopropyl-3, 6-dimethoxybiphenyl-2-yl) phosphine, 3.0 mmol sodium tert-butoxide, and 5 ml toluene, replaced the reaction tube with nitrogen for 3 times, then heated to 120 °C with an oil bath under magnetic stirring, and reacted Reflux for 12 hours. Cool down to room temperature; add to the reaction solution, 3.0 mmol 4-carboxyphenylboronic acid, 0.03 mmol palladium acetate, 0.05 mmol 2'-dicyclohexylphosphino-2,6-di-I-propyl-4-sulfonate- 1,1'-sodium biphenyl, 6.0 mmol sodium carbonate, and 100 mmol water; then heated to 100° C. in an oil bath under magnetic stirring, and the reaction was refluxed for 24 hours. Cool dow...
Embodiment 3
[0029] 2, 2´-N, N-bispyrazolyl biphenyl polycarboxylic acid compound (3): under nitrogen protection, transfer to a 10 ml Schlek reaction tube (a glass commonly used in anhydrous and oxygen-free operation) instrument) by adding 1.0 mmol 2,5-dibromopyrazine, 2.1 mmol 4-iodopyrazole, 0.02 mmol palladium acetate, 0.03 mmol dicyclohexyl (3,6-dimethoxy-2',4',6' -triisopropyl(1,1'-biphenyl)-2-yl)phosphine, 3.0 mmol potassium tert-butoxide, and 5 ml dioxane, replace the reaction tube with nitrogen three times, and then use The oil bath was heated to 110°C and the reaction was refluxed for 18 hours. Cool down to room temperature; then add 5.0 mmol 3,5-dicarboxyphenylboronic acid, 0.03 mmol palladium acetate, 0.05 mmol 2'-dicyclohexyl-2,6-dimethoxy-3-sulfonic acid-1 , 1'-biphenyl sodium salt, 12.0 mmol cesium carbonate, and 200 mmol water; then heated to 110° C. in an oil bath under magnetic stirring, and the reaction was refluxed for 36 hours. Cool down to room temperature; add 20ml ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com