Photosensitive composition
A photosensitive composition and compound technology, applied in the direction of organic chemistry, etc., can solve the problems of insufficient sublimation and pollution of α-aminoalkylphenone derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
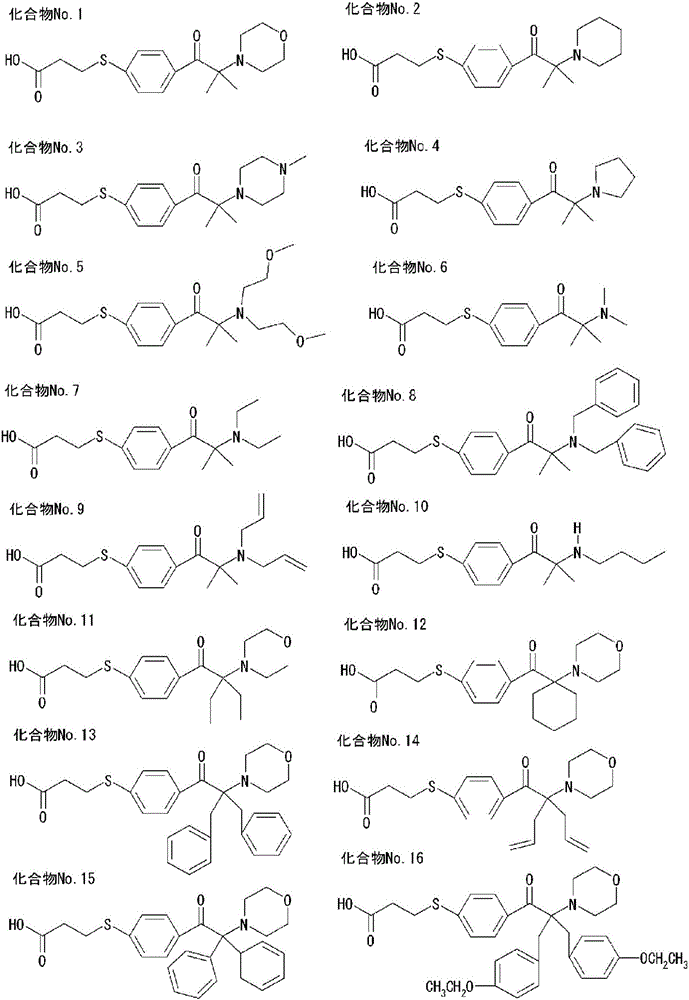
[0146] [Production Example 1] Production of Compound No.1
[0147] 1.0 eq. of halide 1 was dissolved in 9.0 eq. of dimethyl sulfoxide, 4.0 eq. of potassium carbonate and 1.8 eq. of thiol compound 1 were added, and heated and stirred at 70° C. for 10 hours under a nitrogen atmosphere. After cooling to room temperature, it was neutralized with dilute hydrochloric acid and extracted with ethyl acetate. The solvent was distilled off, and the crude product was recrystallized from methanol: ion-exchanged water = 1:1, and dried to obtain Compound No. 1 of [Chem. 3] (yield 23.2%). Various analyzes were performed, and it was confirmed that the obtained compound was target compound No. 1. The analysis results are shown below.
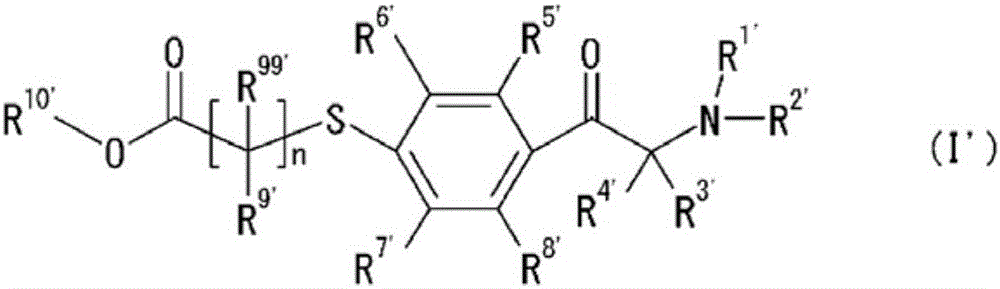
[0148] Furthermore, in this production example, as the halogen body 1, the halogen body 1 in [Chemical 17], R 1’ and R 2’ Linked to form a morpholine ring, R 3’ and R 4’ is methyl, R 5’ , R 6’ , R 7’ and R 8’ is hydrogen, and X is the halogen form of br...
manufacture example 2
[0149] [Production Example 2] Production of Compound No. 17
[0150] 1.0 eq. of halide 1 was dissolved in 9.0 eq. of dimethyl sulfoxide, 4.0 eq. of potassium carbonate and 1.8 eq. of thiol compound 1 were added, and heated and stirred at 120° C. for 20 hours under a nitrogen atmosphere. Add excess toluene, filter and separate impurities, and cool to 5°C. The precipitated solid was filtered and washed. The obtained solid was dissolved in water, neutralized with dilute hydrochloric acid, and extracted from chloroform. The solvent was distilled off, recrystallized from ethyl acetate, and dried to obtain Compound No. 17 (yield 7.6%). Various analyzes were performed, and it was confirmed that the obtained compound was target compound No. 17. The analysis results are shown below.
[0151] Furthermore, in this production example, as the halogen body 1, the halogen body 1 in [Chemical 17], R 1’ and R 2’ Linked to form a morpholine ring, R 3’ and R 4’ is methyl, R 5’ , R 6’ ,...
Embodiment 1
[0161] [Example 1] Manufacture of alkali-developable photosensitive resin composition No.1
[0162] 54.84 g of SPC-1000 (acrylic resin; manufactured by Showa Denko Co., Ltd.), 12.77 g of Aronix M-450 (multifunctional acrylate; manufactured by Toagosei Co., Ltd.), and 0.57 g of KBE-403 (silane coupling agent; manufactured by Shin-Etsu Silicone Co., Ltd.) were mixed , 3.19 g of cyclohexanone 1% solution and 27.95 g of propylene glycol-1-monomethyl ether-2-acetate of FZ-2122 (surfactant; manufactured by Japan Unicar Co., Ltd.), measure 14.805 g from the mixed solution, add 0.195 g of the compound No. 1 obtained in manufacture example 1 was fully stirred, and the alkali-developable photosensitive resin composition No. 1 which is the photosensitive composition of this invention was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com