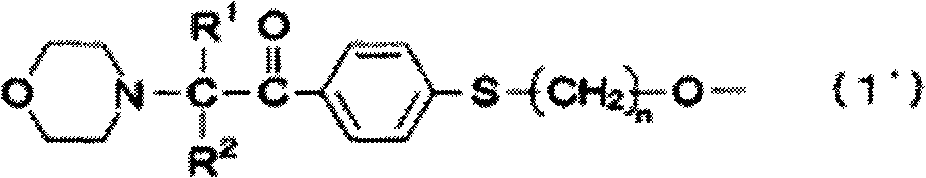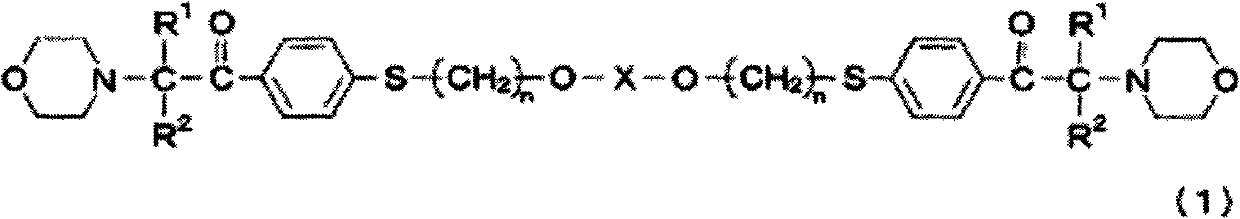Novel compound, process for preparation thereof, radiation -sensitive compositions containing the novel compound, and cured films
A compound and composition technology, applied in the field of radiation-sensitive compositions and cured films, can solve the problems of high exposure, high sublimation, and pollution of photomasks, and achieve high radiation sensitivity, high surface hardness, and low sublimation sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
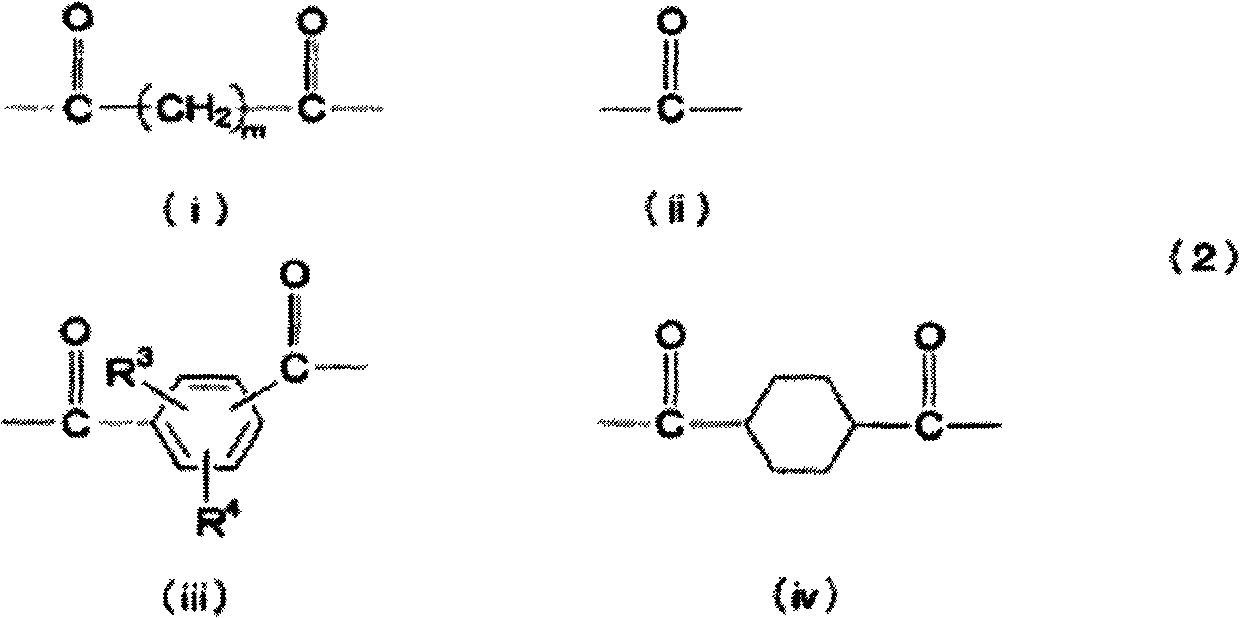
[0161] A thermometer and a dropping funnel were installed in a 1L three-necked flask, and under a nitrogen atmosphere, 110 g of 1-[4-(2-hydroxyethylthio)-phenyl]-2-methyl-2-morpholinyl- Propan-1-one (see Patent Document 4 (US Publication No. 2003-225179)) and 60 g of pyridine were dissolved in 360 g of methylene chloride, and frozen to 5° C. or less in an ice-water bath. Next, a solution obtained by dissolving 33.2 g of adipoyl chloride in 100 g of methylene chloride was dropped over 2 hours through the dropping funnel. After completion of the dropwise addition, the mixture was stirred for 2 hours. After the reaction was finished, 500 mL of water was added to dissolve the generated salt in the water layer, and then the organic layer was extracted using a separatory funnel. The organic layer was washed twice with 200 mL of 10% by mass HCl water, and then twice with 200 mL of distilled water. Anhydrous sodium sulfate was added to the liquid-separated organic layer, water was r...
Embodiment 2
[0167]A thermometer and a dropping funnel were installed in a 1L three-necked flask, and under a nitrogen atmosphere, 110 g of 1-[4-(2-hydroxyethylthio)-phenyl]-2-methyl-2-morpholinyl- Propan-1-one (see Patent Document 4 (US Publication No. 2003-225179)) and 60 g of pyridine were dissolved in 360 g of methylene chloride, and frozen in an ice-water bath to below 5°C. Next, a solution obtained by dissolving 53.8 g of triphosgene in 100 g of methylene chloride was dropped over 2 hours through the dropping funnel. After completion of the dropwise addition, the mixture was stirred for 2 hours. After the reaction was finished, 500 mL of water was added to dissolve the generated salt in the water layer, and then the organic layer was extracted using a separatory funnel. The organic layer was washed twice with 200 mL of 10% by mass HCl water, and then twice with 200 mL of distilled water. Anhydrous sodium sulfate was added to the liquid-separated organic layer, water was removed, an...
Embodiment 3
[0173] A thermometer and a dropping funnel were installed in a 1L three-necked flask, and under a nitrogen atmosphere, 110 g of 1-[4-(2-hydroxyethylthio)-phenyl]-2-methyl-2-morpholinyl- Propan-1-one (see Patent Document 4 (US Publication No. 2003-225179)) and 60 g of pyridine were dissolved in 360 g of methylene chloride, and frozen in an ice-water bath to below 5°C. Next, 42.3 g of 4-methoxyphthaloyl chloride was dissolved in 100 g of methylene chloride through a dropping funnel, and the resulting solution was dropped over 2 hours. After completion of the dropwise addition, the mixture was stirred for 2 hours. After the reaction was finished, 500 mL of water was added to dissolve the generated salt in the water layer, and then the organic layer was extracted using a separatory funnel. The organic layer was washed twice with 200 mL of 10% by mass HCl water, and then twice with 200 mL of distilled water. Anhydrous sodium sulfate was added to the liquid-separated organic layer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com