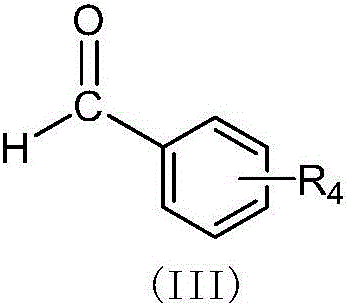
(Z,E)-5-OH-3-(phenyl-derivative-methylene)-dihydroflavone-7-O-glucoside and preparation and application thereof
A dihydroflavonoid, -5-OH-3- technology, applied in the fields of chemistry, cosmetics and medicine, can solve the problems of application limitation and unstable properties, and achieve the effects of single reaction site, easy purification and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Preparation of 3-(4-hydroxyphenylmethine)-hesperidin:
[0040]Weigh 1.0 g of hesperidin with a content of 95%, 0.20 g of p-hydroxybenzaldehyde, add 137 μL of DMSO 3.0 mL tetrahydropyrrole, 100 μL of glacial acetic acid, stir and react at 70 ° C for 24 h, add silica gel to mix the sample, wash with chloroform-methanol gradient After removal, 775 mg of yellow powder was obtained, which was 3-(4-hydroxybenzylmethine)-hesperidin (cpd 1). 1 H NMR (400 MHz, DMSO) δ12.63 (d, 1H: 5-OH), 10.27 (s, 1H: 3'-OH), 9.17 (s, 1H: 15-OH), [8.32+7.94 (d ,1H:11-H)], 7.27-7.31(2H:13-H+17-H), 6.75-7.00(m,5H:14-H+16-H+2'-H+5'-H+ 6'-H), 6.66 (d, 1H: 2-H), 6.05-6.12 (dd, J=2.0, 2H: H-6+H-8), 3.74 (s, 3H: 4'-OCH 3 ), 5.36 (1H: Gly-1"), 4.90 (1H: Rha-1"'), 1.10 (dd,3H: Rha-6"'); 13 C NMR (100 MHz, DMSO-d6) δ185.81 (d, C-4), 165.97 (d, C-7), 164.34 (C-9), 160.46 (C-5), 148.55 (C-4' ), 147.75(C-3'), 133.42(C-1'), 130.38(C-3), 118.93(C-6'), 115.01(C-2'), 112.85(C-5'), 103.94 (C-10...
Embodiment 2
[0042] Example 2: Preparation of 3-(3,4-hydroxyphenylmethine)-hesperidin:
[0043] Weigh 1.5 g of hesperidin with a content of 95%, add 200 μL of tetrahydropyrrole and 4.0 mL of DMSO to dissolve, add 150 μL of glacial acetic acid, stir and react at 60°C for 3 h, then add 0.25 g of 3,4-hydroxybenzaldehyde in portions, The addition was completed within 8 hours, and the reaction was continued for 3 hours. The sample was mixed with silica gel and eluted with a gradient of chloroform-methanol to obtain 803 mg of yellow powder, which was 3-(3,4-hydroxybenzylmethine)-hesperidin (cpd 2). 1 H NMR (400 MHz, DMSO) δ12.63 (d, 1H: 5-OH), 9.17 (s, 1H: 3'-OH), [8.31+7.85 (d, 1H: 11-H)], 6.99– 6.70(m,6H:2'-H+5'-H+6'-H+13-H+16-H+17-H), 6.56(d,1H:2-H), 6.09(d,2H : 6-H+8-H), 5.37(s, 1H: 2-H), 3.74(s, 3H: 4'-OCH 3 ); 5.37 (s, 1H: Gly-1"'-H), 4.95 (d, 1H: Rha-1"'-H), 1.10 (d, 3H: Rha-6"'). 13 C NMR (100 MHz, DMSO-d6) δ185.79 (C-4), 165.92 (C-7), 164.34 (C-9), 160.20 (C-5), 149.21 (C-4'), 148.51...
Embodiment 3
[0045] Example 3: Preparation of 3-(3,4-hydroxyphenylmethine)-naringin
[0046] Weigh 1.5 g of naringin with a content of 98%, add 200 μL of tetrahydropyrrole and 4.0 mL of DMSO to dissolve, add 150 μL of glacial acetic acid, stir and react at 60°C for 3 hours, then add 0.30 g of 3,4-hydroxybenzaldehyde in portions, The addition was completed within 8 hours, and the reaction was continued for 3 hours. The sample was mixed with silica gel and eluted with a gradient of chloroform-methanol to obtain 845 mg of yellow powder, which was 3-(3,4-hydroxybenzylmethine)-naringin (cpd 3). 1H NMR (400 MHz, DMSO) δ12.70(d,1H:5-OH), 9.65(br,1H:15-OH), 9.13(br,1H:14-OH), [8.31+7.85(d, 1H: 11-H)], 7.17-7.30 (2H: 13-H+17-H), 6.68-6.90 (m, 5H: 2'-H+3'-H+5'-H+6'-H +16-H), 6.50-6.65(d,1H:2-H), 5.32(1H:2-H); 5.0-5.20(2H:Gly-1"'-H+Rha-1"'-H) 1.17 (d,3H:Rha-6"'). 13 C NMR (100 MHz, DMSO-d6) δ185.76 (C-4), 165.27 (C-7), 164.14 (C-9), 160.12 (C-5), 158.24 (C-4'), 133.48 C -3), 129.23(C-1'), 128.93(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com