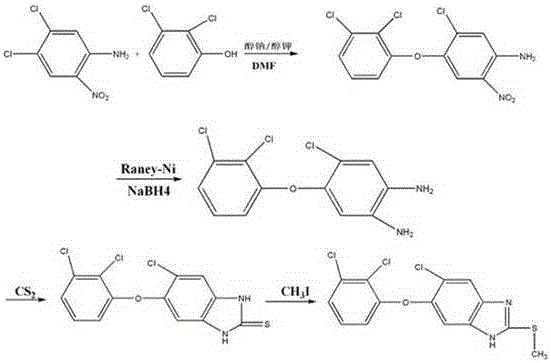
Method for preparing triclabendazole serving as medicine for animal distomiasis
A technology for triclabendazole and flukes, which is applied in the field of preparation of new veterinary drugs, can solve the problems of low reaction yield, complicated preparation process, and "three wastes" to achieve high reaction efficiency, high reaction yield, and low process cost. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (1) Preparation of potassium methoxide
[0020] Add 200mL of petroleum ether (pre-dried) and 46.8g (1.2mol) of potassium metal into a 2L reactor, add about 200mL of anhydrous methanol dropwise under stirring, and the dropping speed depends on the solution temperature and H 2 Depends on the amount released. Reflux for 0.5 h after the addition, and cool to room temperature to obtain a white slurry potassium methoxide solution for later use.
[0021] (2) Preparation of 4-chloro-5-(2,3-dichlorophenoxy)-2-nitroaniline
[0022] Under the microwave radiation condition of 1000W, the potassium methoxide solution prepared in step (1) (1.2mol of potassium methoxide), 2,3-dichlorophenol (1mol), 3,4-dichloro-5-nitroaniline (0.9 mol) and 20ml of DMF solvent into the reactor, 2,3-dichlorophenol, 3,4-dichloro-5-nitroaniline, sodium alkoxide or potassium alkoxide solution were fully stirred and mixed in the reactor, under nitrogen atmosphere , heated to 155°C for full reaction. After...
Embodiment 2
[0030] (1) Preparation of potassium methoxide
[0031] Add 200mL of petroleum ether (pre-dried) and 42.9g (1.1mol) of metal potassium into a 2L reactor, add about 200mL of anhydrous methanol dropwise under stirring, and the dropping speed depends on the solution temperature and H 2 Depends on the amount released. Reflux for 0.5 h after the addition, and cool to room temperature to obtain a white slurry potassium methoxide solution for later use.
[0032] (2) Preparation of 4-chloro-5-(2,3-dichlorophenoxy)-2-nitroaniline
[0033] Under the microwave radiation condition of 1000W, the potassium methoxide solution prepared in step (1) (potassium methoxide 1.1mol), 2,3-dichlorophenol (1mol), 3,4-dichloro-5-nitroaniline (0.9 mol) and 20ml of DMF solvent into the reactor, 2,3-dichlorophenol, 3,4-dichloro-5-nitroaniline, sodium alkoxide or potassium alkoxide solution were fully stirred and mixed in the reactor, under nitrogen atmosphere , heated to 150°C for full reaction. After fu...
Embodiment 3
[0041] The reaction conditions of other step 1 are the same as those of Example 2, only the etherification reaction of step 2 is reacted at a temperature of 160°C, the reaction time is 20min, the reaction raw materials are completely reacted, and the yellow solid 4-chloro- 5-(2,3-dichlorophenoxy)-2-nitroaniline 323.7g, yield 97.5%, HPLC purity 98.9%.
[0042] (3) Preparation of 4-chloro-5-(2,3-dichlorophenoxy)-1,2-phenylenediamine
[0043] Add 4-chloro-5-(2,3-dichlorophenoxy)-2-nitroaniline (1 mol), 1 mol of 10% Pd / C catalyst and 1.6 mol of sodium borohydride and 100 ml of anhydrous toluene into the reactor , heated to reflux, fully reacted for 2 hours, cooled to room temperature, filtered, concentrated under reduced pressure to recover the reaction solvent, added petroleum ether, and crystallized to obtain 4-chloro-5-(2,3-dichlorophenoxy)-1 , 284.2g of 2-phenylenediamine, yield 94.1%, purity 99.0%.
[0044] (4) Preparation of 4-chloro-5-(2,3-dichlorophenoxy)-1H-benzimidazol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com