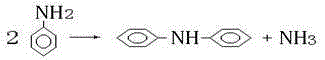
Method for improving conversion rate of aniline in synthesis of diphenylamine
A technology for the conversion rate of diphenylamine and aniline, which is applied in the fields of chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of low conversion rate of diphenylamine, achieve the improvement of conversion rate of aniline, increase conversion rate, experimental method simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Catalyst preparation: The catalyst used in the experiment is a catalyst composed of beta zeolite, activated alumina and alkali metal. The specific preparation method is as follows:
[0030] (1) Take 200g Hβ zeolite (SiO 2 / Al 2 o 3 The molecular ratio is 28) and 1500mL (0.1mol / L) potassium chloride aqueous solution, placed in a 2000mL four-neck flask with a stirrer for ion exchange. The exchange reaction temperature is 75°C, the stirring speed is 200n / min, and the time is 5.0h. Afterwards, the above-mentioned exchanged zeolite and potassium aqueous solution are filtered, washed and dried; the washing process is: washing until no chlorine is present. The drying process is as follows: drying at 60° C. for 4.0 hours, and drying at 110° C. for 4.0 hours.
[0031] (2) Mix 70g of the above exchanged zeolite with 10g of aluminum hydroxide powder, add nitric acid and an appropriate amount of deionized water, wherein the amount of nitric acid added accounts for 0.5wt% of th...
Embodiment 2~4
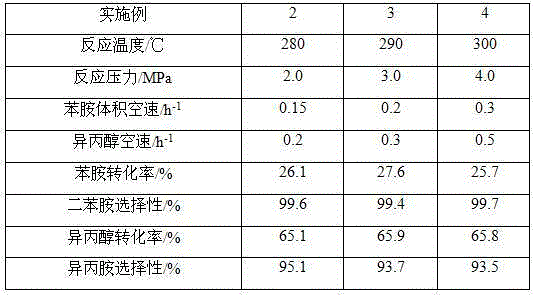
[0033] A fixed bed reactor was adopted, and the catalyst prepared in Example 1 was used, and 200 mL of the catalyst was taken, and loaded into a stainless steel reactor with an inner diameter of 25 mm and a length of 1200 mm. The purity of the reaction raw material aniline is >99%, and the purity of isopropanol is >99%. Using the bottom feeding method, the aniline and isopropanol are fed into the reactor at the same time, and the synthesis reaction is carried out at different reaction temperatures, pressures and feed space velocities. The reaction products flow out from the top of the reactor and enter the separator after cooling. middle. The composition was analyzed by gas chromatography, and the specific reaction conditions and results are shown in Table 1.
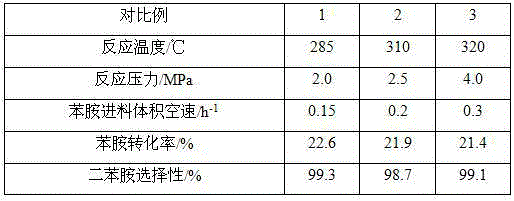
[0034] The reaction conditions and the result of table 1. embodiment 2~4
[0035]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com