Method for synthesizing 1,2,3-triazole compound through one-pot two-step method
A compound and triazole technology, which is applied in the field of synthesis of 1,2,3-triazole compounds, can solve the problems of low yield, poor selectivity, cumbersome reaction steps and the like, and achieves simple process, convenient operation and simplification The effect of post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Using different monovalent copper salts as catalysts, 1.0mmol iodobenzene, 1.2mmol sodium azide, 0.3mmol DBU and 0.2mmol catalyst were sequentially added to a reaction flask containing 5.0mL dimethyl sulfoxide, and the reaction mixture Stir the reaction at 95°C, track the reaction until the reaction is complete by TLC, add 1.0mmol phenylacetaldehyde (or phenylacetylene) to the reaction bottle after the reaction is cooled to room temperature, and add 50mL water to the reaction mixture and add a few drops of Ammonia, then extracted 3 times with dichloromethane, washed 1 time with saturated brine, dried, filtered with suction, removed solvent, and purified by column chromatography to obtain the target product. The influence of different catalysts on the reaction yield is shown in Table 1.
[0019] Table 1 Effect of different catalysts on reaction yield
[0020] Group catalyst temperature (℃) Reaction time (h) Yield (%) 1 CuI 95 / r.t. 2.5 / 0.5 92 ...
Embodiment 2
[0022] Add 1.0mmol of iodobenzene, 1.2mmol of sodium azide, 0.3mmol of DBU and 0.2mmol of cuprous iodide to a reaction flask containing 5.0mL of dimethyl sulfoxide, and stir the reaction mixture at 95°C for 2.5h , TLC followed the reaction until the reaction was complete. When the reaction was cooled to room temperature, 1.0mmol phenylacetaldehyde (or phenylacetylene) was added to the reaction bottle. After 0.5h of reaction, 50mL water was added to the reaction mixture and a few drops of ammonia water were added, and then Extracted three times with dichloromethane, washed once with saturated brine, dried, filtered with suction, spin off the solvent, and purified by column chromatography to obtain the target product with a yield of 92%.
[0023]
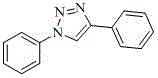
[0024] Data characterization of 1,4-diphenyl-1,2,3-triazole: off-white crystals; 1 H NMR (400 MHz, CDCl 3 ) δ : 8.20 (s, 1H), 7.95-7.89 (m, 2H), 7.82-7.77 (m, 2H), 7.55 (t, J = 7.7Hz,2H), 7.50-7.43 (m, 3H), 7.38 (t, J = 7.4 ...
Embodiment 3
[0026] Add 1.0mmol of iodobenzene, 1.2mmol of sodium azide, 0.1mmol of DBU and 0.1mmol of cuprous iodide to a reaction flask containing 5.0mL of dimethyl sulfoxide, and stir the reaction mixture at 95°C for 2.5h , TLC followed the reaction until the reaction was complete. When the reaction was cooled to room temperature, 1.0mmol phenylacetaldehyde (or phenylacetylene) was added to the reaction bottle. After 0.5h of reaction, 50mL water was added to the reaction mixture and a few drops of ammonia water were added, and then Extracted three times with dichloromethane, washed once with saturated brine, dried, filtered with suction, spin off the solvent, and purified by column chromatography to obtain the target product with a yield of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com