Pharmaceutical composition for controlling citrus gonggan canker
A technology of canker and composition, which is applied in the field of pharmaceutical composition for preventing and treating citrus canker, can solve the problems of low yield and sugar content rate of tribute citrus, general taste and appearance color, short drug effect time, etc., and achieve effective Conducive to popularization and application, the appearance is round and lustrous, and the effect of long-term drug effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] The preparation method of described prevention and treatment tribute orange canker medicine composition, comprises the following steps:
[0056] S1: Mix and pulverize purslane, tea leaves, pyrethrum, lettuce, weeping willow leaves, kava pepper, tea leaves, and leafless horsetail to obtain mixture I. Add mixture I to ethanol, and the ratio of solid to liquid is 1 : 14-16, the ultrasonic power is 200-300W, the ethanol concentration is 65%-80%, and the extraction temperature is 64-76°C to extract the medicinal solution for 17-23min to obtain the medicinal solution;
[0057] S2: passing the liquid medicine prepared in step S1 through a 3000-5000 mesh sieve to obtain a filtrate;
[0058] S3: adding potassium salt, phosphorus salt, nitrogen salt, calcium salt, magnesium salt, iodine salt, boron salt, molybdenum salt, zinc salt, manganese salt, copper salt, iron salt, vitamin, amino acid, Indolebutyric acid, gibberellin, 6-benzyl adenine, naphthaleneacetic acid, agar, kinetin...
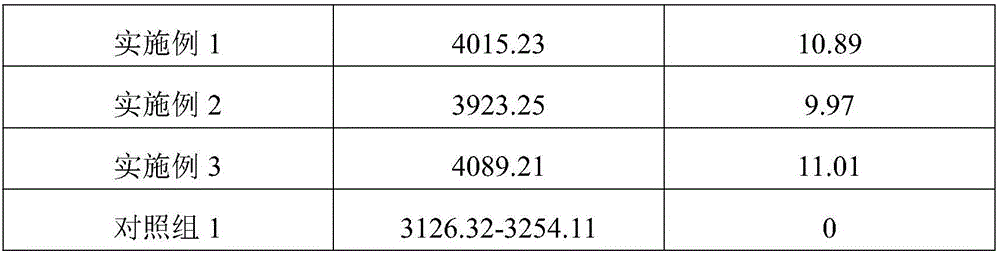
Embodiment 1
[0064] A pharmaceutical composition for preventing and treating citrus canker, in parts by weight, comprising the following raw materials: 126 parts of purslane, 100 parts of tea tree leaves, 90 parts of pyrethrum, 90 parts of lettuce, 75 parts of weeping willow leaves, 65 parts of kava 55 parts of tea tree leaves, 50 parts of leafless horsetail, 2.5 parts of potassium salt, 2 parts of phosphorus salt, 1.5 parts of nitrogen salt, 0.8 part of calcium salt, 0.8 part of magnesium salt, 0.3 part of iodine salt, 0.3 part of boron salt, 0.3 part of molybdenum salt, 0.3 part of zinc salt, 0.3 part of manganese salt, 0.3 part of copper salt, 0.3 part of iron salt, 0.3 part of vitamin, 0.2 part of amino acid, 0.08 part of indole butyric acid, 0.08 part of gibberellin, 6-benzyl 0.07 parts of adenine, 0.05 parts of naphthalene acetic acid, 0.2 parts of agar, 0.05 parts of kinetin, 1.5 parts of N-methylpyrrolidone, 1 part of sodium dinonyl naphthalene sulfonate, 1.4 parts of DuPont EEA2112...
Embodiment 2
[0089] A pharmaceutical composition for preventing and treating citrus canker, comprising the following raw materials in parts by weight: 112 parts of purslane, 92 parts of tea tree leaves, 82 parts of pyrethrum, 82 parts of lettuce, 60 parts of weeping willow leaves, 55 parts of kava 45 parts of tea tree leaves, 40 parts of leafless horsetail, 2 parts of potassium salt, 1 part of phosphorus salt, 1 part of nitrogen salt, 0.6 part of calcium salt, 0.6 part of magnesium salt, 0.2 part of iodine salt, 0.2 part of boron salt, 0.2 part of molybdenum salt, 0.2 part of zinc salt, 0.2 part of manganese salt, 0.2 part of copper salt, 0.2 part of iron salt, 0.2 part of vitamin, 0.2 part of amino acid, 0.06 part of indole butyric acid, 0.06 part of gibberellin, 6-benzyl 0.06 part of adenine, 0.04 part of naphthalene acetic acid, 0.1 part of agar, 0.04 part of kinetin, 0.7 part of N-methylpyrrolidone, 0.6 part of sodium dinonyl naphthalene sulfonate, 1 part of American DuPont EEA2112AC co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com