Novel method for preparing dolutegravir
A technology of dolutegravir and a new method, which is applied in the field of preparation of dolutegravir, can solve the problems of expensive materials, high cost, instability, etc., and achieve the effects of simple operation, improved utilization rate, and improved total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
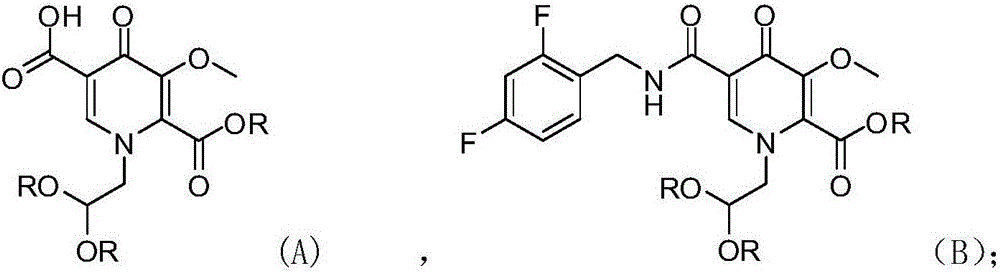
[0029] Example 1 1-(2,2-dimethoxyethyl)-1,4-dihydro-3-methoxy-4-oxo-5-(2,4-difluorobenzaminoyl) Preparation of pyridine-2-methyl ester (compound B)
[0030]
[0031] Put tetrahydrofuran (700g), compound A (80g, 0.254mol) and CDI (49.4g, 1.2eq) into the reaction flask; heat up to reflux (70°C), stir for 2.5h; put in CDI (12.4g, 0.3eq), continue Reflux and stir for 2.0h; cool down to 20-25°C, add 2,4-difluorobenzylamine (40.0g, 1.1eq); maintain 20-25°C and stir for 5h; after the reaction is complete, add methyl tert-butyl ether ( 600g) and 3% hydrochloric acid (600g); Stir for 10 minutes, leave standstill for 10 minutes; Layer, organic layer drops into 3% sodium hydroxide (600g); Stir for 10 minutes, leave standstill for 10 minutes; Layer, organic layer drops into water (600g); Stir for 10 minutes, leave standstill for 10 minutes; separate layers, concentrate the organic phase to dryness, and directly carry out the next step reaction.
Embodiment 2
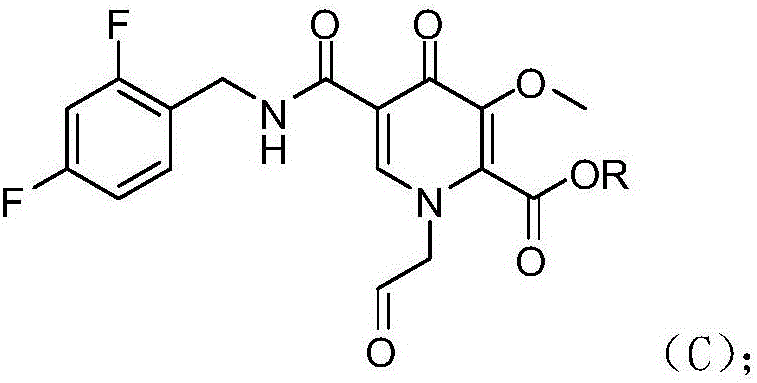
[0032] Example 2 1-(2-oxoethyl)-1,4-dihydro-3-methoxy-4-oxo-5-(2,4-difluorobenzyl)pyridine-2- Preparation of Methyl Ester (Compound C)
[0033]
[0034] Add formic acid (240g) to the concentrate obtained in Example 1; heat up to 60°C, stir for 3 hours; steam the formic acid under reduced pressure, drop in 10% sodium dihydrogen phosphate (600g) and dichloromethane (800g); stir for 10 Minutes, let stand for 10 minutes; Layered, the aqueous layer was extracted once with dichloromethane (400g); Layered, combined organic layer, after evaporating the solvent, drop into methyl tert-butyl ether (240g); Stir at room temperature for 3h, filter , Compound C was collected as a white solid with a yield of 92%.
Embodiment 3
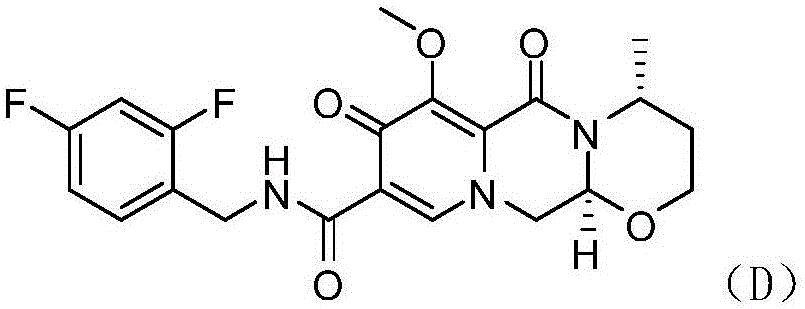
[0035] The preparation of embodiment 3 degree lutevir
[0036]
[0037]Put acetonitrile (700g) and compound C (90g, 0.228mol) into the reaction flask; stir and heat up to 50°C, add acetic acid (13.7g, 1eq); heat up to 60-65°C, add dropwise R-3-amino-1 - Butanol (22.3g, 1.1eq) and acetonitrile (100g); Insulated and stirred reaction 15h to the HPLC purity of compound (D) in the liquid phase is 98.0% after dropping into magnesium bromide hexahydrate (199.7g, 3eq), warming up to 80°C, stirred for 2 hours; after the reaction was completed, 3% hydrochloric acid (830g) and dichloromethane (1200g) were added; layered, the water layer was dropped into dichloromethane (600g); layered, the combined organic layers were concentrated to dryness, and ethanol was added , and concentrated to dryness again to obtain dolutegravir (yield 90.9%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com