Preparation method of rivastigmine tartrate
A technology of rivastigmine bitartrate and ethyl acetate, which is applied in the field of medicine and chemical industry, can solve the problems of low purity and low yield of rivastigmine bitartrate, achieve improved yield and purity, simple operation, and is suitable for industrial production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
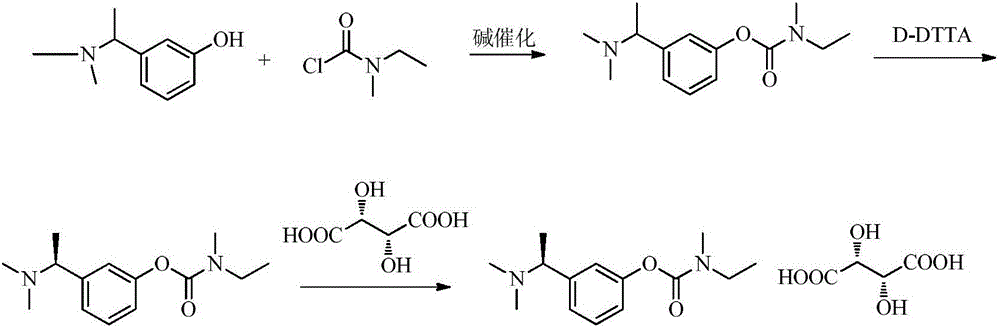
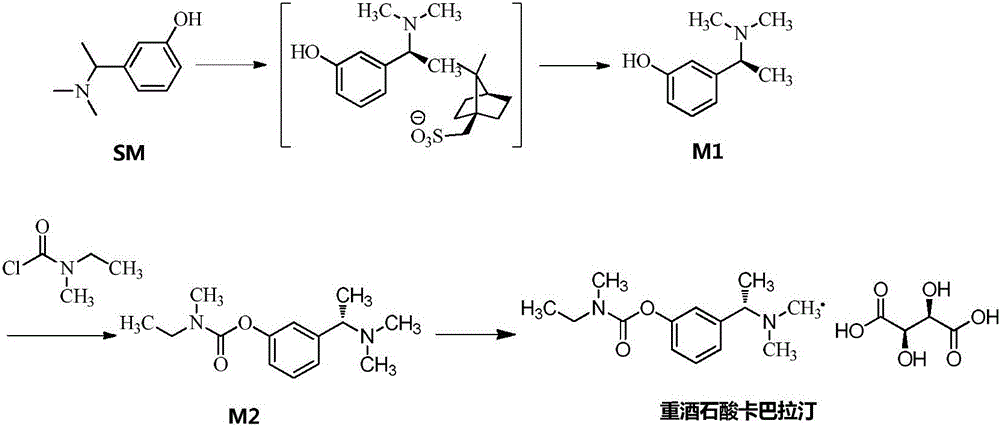
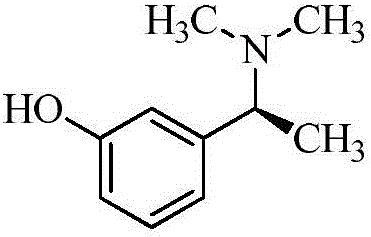
[0080] A. Intermediate 1: Synthesis of (S)-3-(1-(dimethylamino)ethyl)phenol
[0081]
[0082]Add 1L of ethyl acetate and 200.0g of (R,S)-3-(1-(dimethylamino)ethyl)phenol as raw materials into the reaction flask, stir and dissolve at 40°C, and dissolve the prepared S-(+) - Camphorsulfonic acid ethanol (600ml) solution (dissolve 235 grams of S-(+)-camphorsulfonic acid in 600 milliliters of ethanol) into the above-mentioned reaction flask, stir and reflux for 30min, cool to room temperature after the system dissolves, and then cool to 0 ° C, stirred and crystallized for 2h.
[0083] Suction filtration and drying gave 235.9 g of white solid. Add the obtained solid to the reaction flask, then add 500ml of ethanol and 900ml of ethyl acetate, stir and reflux, cool to room temperature naturally after 30min, then cool down to 0°C to crystallize for 2h, and filter with suction to obtain 182.7g of solid.
[0084] The obtained 182.7g solid was dissolved in 600ml of water, stirred and...
Embodiment 2
[0092] A. Intermediate 1: Synthesis of (S)-3-(1-(dimethylamino)ethyl)phenol
[0093]
[0094] Add 0.8L ethyl acetate and 180.0g (R,S)-3-(1-(dimethylamino)ethyl)phenol as raw materials into the reaction flask, stir and dissolve at 30°C, and mix the prepared S-(+ )-camphorsulfonic acid ethanol (480ml) solution (dissolve 170 grams of S-(+)-camphorsulfonic acid in 450 milliliters of ethanol) into the above-mentioned reaction flask, stir and reflux for 25min, cool to room temperature after the system dissolves, and then cool To 0 ℃, stirring crystallization 1.5h.
[0095] Suction filtered and dried to obtain a white solid. Add the obtained solid to the reaction flask, then add 450ml of ethanol and 750ml of ethyl acetate, stir and reflux, cool to room temperature naturally after 25min, cool down to 0°C to crystallize for 1.5h, and filter to obtain the solid.
[0096] The obtained solid was dissolved in 550ml of water, stirred to dissolve, and the insoluble matter was removed by...
Embodiment 3
[0103] A. Intermediate 1: Synthesis of (S)-3-(1-(dimethylamino)ethyl)phenol
[0104]
[0105] Add 2L of ethyl acetate and 300.0g of (R,S)-3-(1-(dimethylamino)ethyl)phenol as raw materials into the reaction flask, stir and dissolve at 50°C, and dissolve the prepared S-(+) - Camphorsulfonic acid ethanol (600ml) solution (dissolve 350 grams of S-(+)-camphorsulfonic acid in 850 milliliters of ethanol) into the above-mentioned reaction flask, stir and reflux for 40min, cool to room temperature after the system dissolves, and then cool to 0 ℃, stirring crystallization 3h.
[0106] Suction filtration, drying to obtain a white solid, the obtained solid was added to a reaction flask, and then 700ml of ethanol and 1100ml of ethyl acetate were added, stirred and refluxed, cooled to room temperature naturally after 40min, and then cooled to 0°C for 3 hours of crystallization, and suction filtered to obtain solid g.
[0107] The obtained solid was dissolved in 800ml of water, stirred ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com