Lithium ion battery electrolyte and lithium ion battery
A lithium-ion battery and electrolyte technology, applied in the field of electrochemistry, can solve problems such as hindering the application of flame retardant additives, increasing the viscosity of the electrolyte, reducing the conductivity of the electrolyte, etc., and achieves the effect of small negative impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0034] Preparation Example 1: Preparation of Cyclohexylphenoxypentafluorotripolyphosphazene
[0035] Using cyclohexylphenol and hexafluorocyclotriphosphazene as raw materials, triethylamine is selected as the acid-binding agent, fed into the organic solvent (acetonitrile) in the autoclave at one time, stirred rapidly, heated to 90°C, and the reaction was completed in 4 hours. After cooling to room temperature, it was filtered to obtain cyclohexylphenoxypentafluorotrimeric phosphazene.
[0036] The reaction equation is:
[0037]
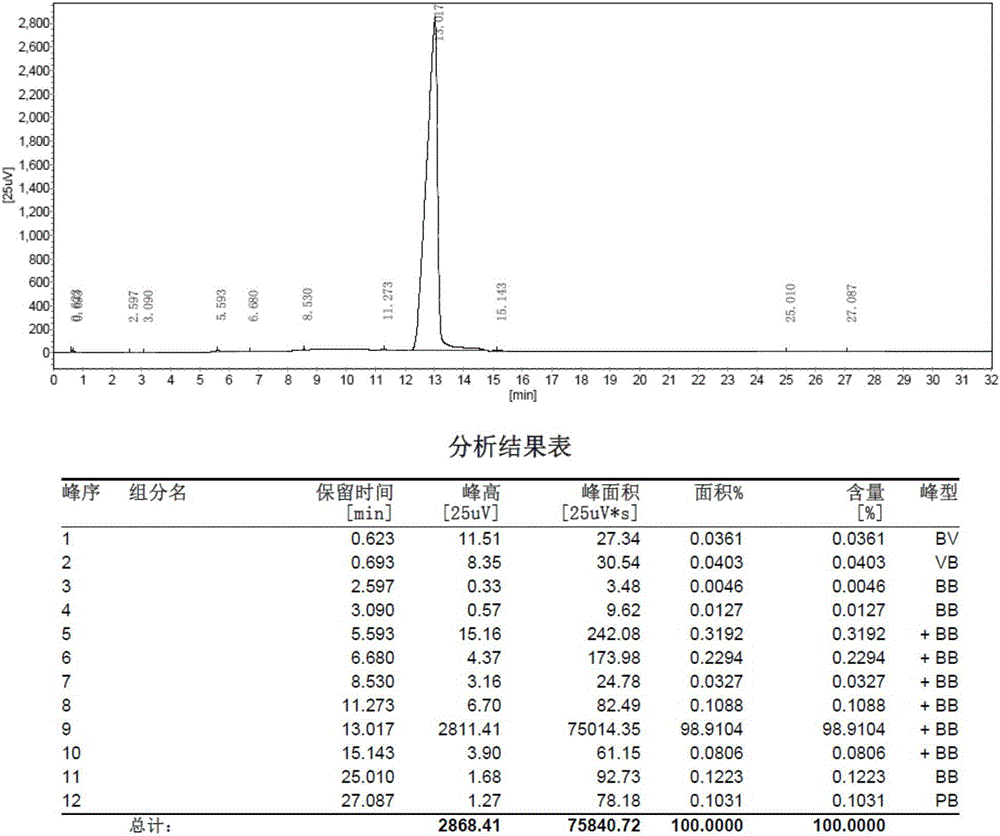
[0038] For the MS chart of cyclohexylbenzenepentafluorotripolyphosphazene, see figure 1 , GC diagram see figure 2 , TCD diagram see image 3 .
preparation example 2
[0039] Preparation 2: tert-butylphenoxypentafluorotripolyphosphazene
[0040]Use p-tert-butylphenol and hexafluorocyclotriphosphazene as raw materials, select triethylamine as the acid-binding agent, feed in the organic solvent (acetonitrile) in the autoclave at one time, stir rapidly, heat up to 90°C, and react for 4 hours Finish. After cooling to room temperature, tert-butylphenoxypentafluorotripolyphosphazene was obtained by filtration.
preparation example 3
[0041] Preparation 3: 3,4-Difluorobiphenoxypentafluorotripolyphosphazene
[0042] Use 3,4-difluorobiphenol and hexafluorocyclotriphosphazene as raw materials, select triethylamine as the acid-binding agent, feed in the organic solvent (acetonitrile) in the autoclave at one time, stir rapidly, and heat up to 90°C. The reaction was completed in 4 hours. After cooling to room temperature, 3,4-difluorobiphenoxypentafluorotripolyphosphazene was obtained by filtration.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com