High-sensitivity BHV-2 (bovine herpes virus 2) quantitative real-time PCR (polymerase chain reaction) detection method and kit
A real-time fluorescence quantitative, BHV-2-P technology, applied in the determination/inspection of microorganisms, biochemical equipment and methods, DNA/RNA fragments, etc. High-accuracy detection requirements, the sensitivity is not as good as real-time quantitative PCR and other problems, to achieve the effect of high accuracy, low cross-hybridization interference, and improved repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1 A kind of kit for detecting bovine herpes virus type 2
[0061] The kit includes:
[0062] (1) Primer BHV-2-F1, the nucleotide sequence of which is shown in SEQ ID NO: 1;
[0063] (2) Primer BHV-2-R1, the nucleotide sequence of which is shown in SEQ ID NO: 2;
[0064] (3) Primer BHV-2-F2, the nucleotide sequence of which is shown in SEQ ID NO: 3;
[0065] (4) Primer BHV-2-R2, the nucleotide sequence of which is shown in SEQ ID NO: 4;
[0066] (5) Probe BHV-2-P, the nucleotide sequence of which is shown in SEQ ID NO: 5, the 5' end of the nucleotide sequence is connected to the fluorescent group FAM, and the 3' end is connected to the quenching group BHQ1;
[0067] (6) Positive quality control product: containing the genomic DNA of bovine herpesvirus type 2 as a PCR template, with primers BHV-2-F1 and BHV-2-R1 as the plasmid of the DNA fragment amplified by PCR primers, the plasmid concentration is 1.0×10 8 copy / μL;
[0068] (7) Negative quality control...
Embodiment 2
[0069] Embodiment 2 A kind of method for detecting bovine herpes virus type 2
[0070] The detection kit used is the kit described in Embodiment 1 of the present invention.
[0071] Samples to be tested: 30 bovine blood or skin tissues from different sources, known to be infected with BHV-2, collected from the Animal Disease Prevention and Control Center of Anlu City, Hubei Province, sample numbers 1-30.
[0072] (1) Extract viral DNA from the samples to be tested: use the viral genomic DNA / RNA extraction kit (purchased from Tiangen Biochemical Technology Co., Ltd., article number is DP315) to extract viral genomic DNA from 30 samples as the DNA to be tested. For the extraction method, please refer to the instruction manual of the extraction kit;
[0073] (2) Preparation of positive quality control products:
[0074] 1) With the genomic DNA of BHV-2 as PCR template, carry out PCR amplification with primer BHV-2-F1-1 and BHV-2-R1-1 as PCR primers:
[0075] The PCR system is ...
experiment example 1
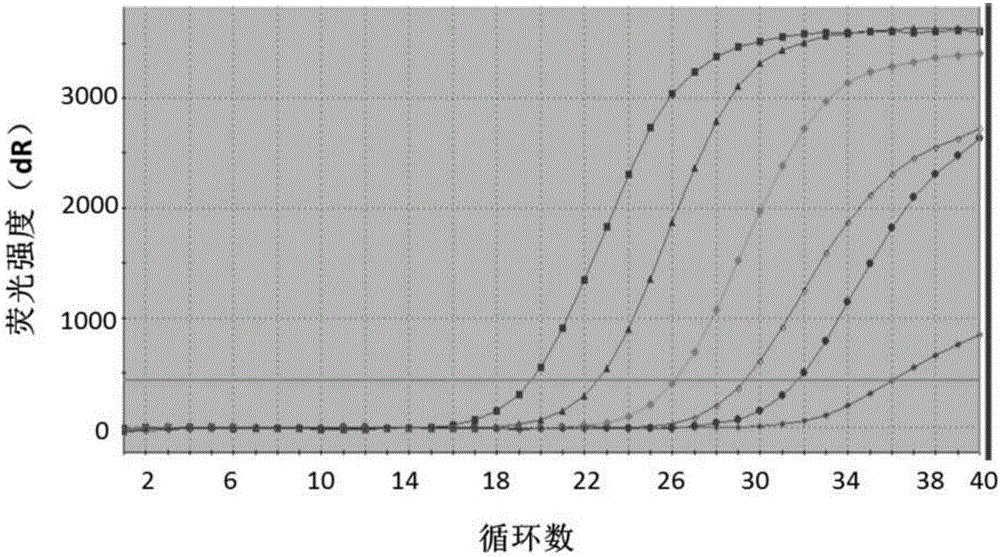
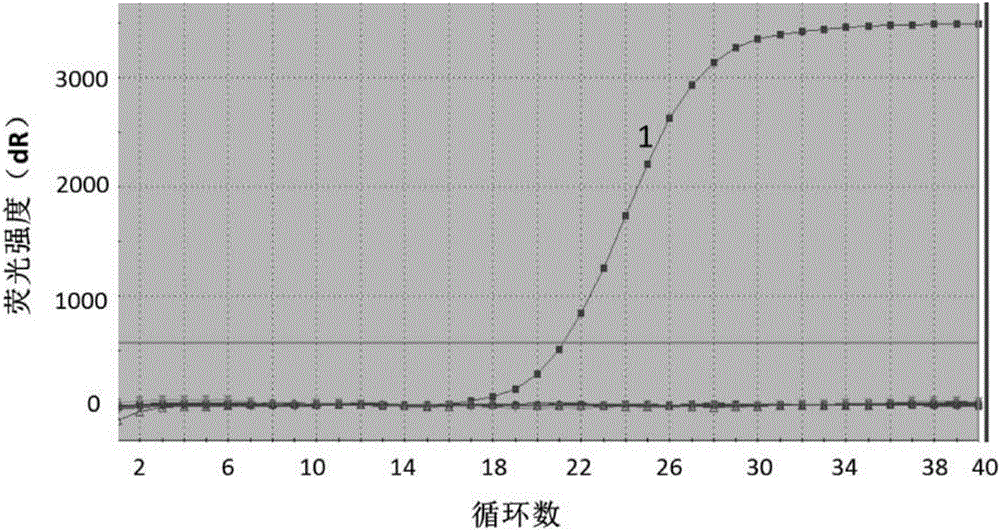
[0102] Experimental Example 1 Sensitivity Experiment
[0103] The positive quality control product described in the present invention was diluted to 1.0×10 7 , 1.0×10 6 , 1.0×10 5 , 1.0×10 4 , 1.0×10 3 , 1.0×10 2 , 1.0×10 1 Copy / mL, as the sample to be detected, the sensitivity experiment was carried out on the detection method of Example 2, and the sensitivity was characterized by the lowest detection concentration. figure 1 For the sensitivity test results, according to figure 1 It can be seen that the plasmid copy number is 10 8 ~10 2 In the copy / mL range, there are curves with obvious exponential growth period, and the plasmid copy number is 10 2 copies / mL, there is still a fluorescent signal through amplification, so the detection sensitivity of the method provided by the invention is: 1.0×10 2 copies / mL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com