Levorotatory oxiracetam slow-release capsule with good releasing rate uniformity and preparation method thereof
A technology of sustained-release capsules and uniformity, which is applied in the directions of non-active ingredient medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc. Achieving problems such as sustained-release preparations, achieving the effects of good content uniformity of the main drug, simple and feasible preparation process, and reducing the number of doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A levooxiracetam sustained-release capsule with good release uniformity is prepared according to the following steps:
[0023] Element Dosage Levooxiracetam 1 serving lactose 1.3 servings Hypromellose K4M 1.0 servings Hypromellose K15M 1.5 servings carnauba wax 0.5 servings octadecanol 0.08 servings
[0024] 70% ethanol solution 2.3 servings
[0025] 1000 capsules
[0026] Formulation process:
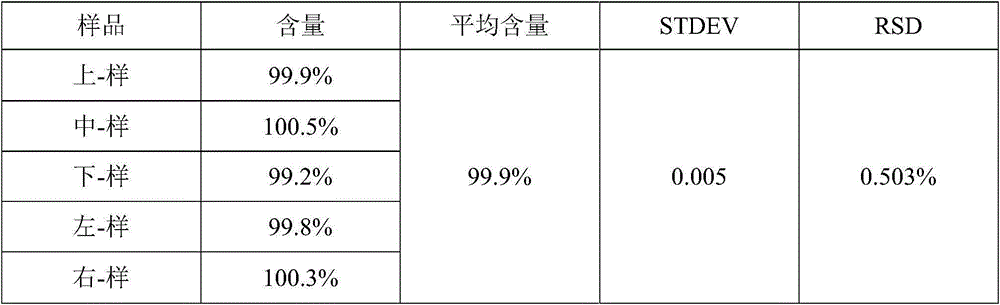
[0027] 1. Pretreatment of raw and auxiliary materials: Dissolve L-Oxiracetam into a solution with 3 to 7 times the amount of water for use; take the formulation amount of slow-release matrix material and blocker, add L-Oxiracetam aqueous solution, stir and mix 10min~15min, put in blast drying oven, set temperature 40℃~60℃, dry to moisture ≤3%, take out, put in mixing pulverizer and mix and pulverize into fine powder (all pass through No. 5 sieve and can pass 6 The amount of No. sieve shall not be less th...
Embodiment 2
[0068] A levooxiracetam sustained-release capsule with good release uniformity is prepared according to the following steps:
[0069] Element Dosage Levooxiracetam 1 serving lactose 1.5 servings Carboxypropyl methylcellulose K4M 1.3 servings Carboxypropyl methylcellulose K15M 1.8 servings carnauba wax 0.7 servings octadecanol 0.18 servings 70% ethanol solution 2.7 servings
[0070] 1000 capsules
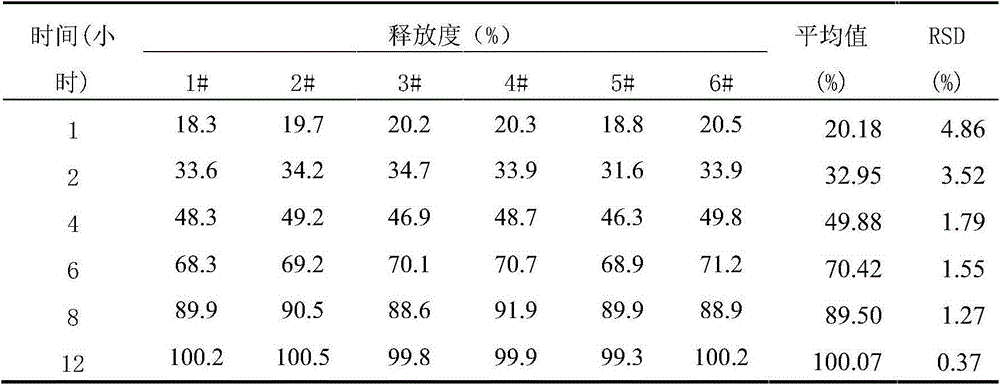
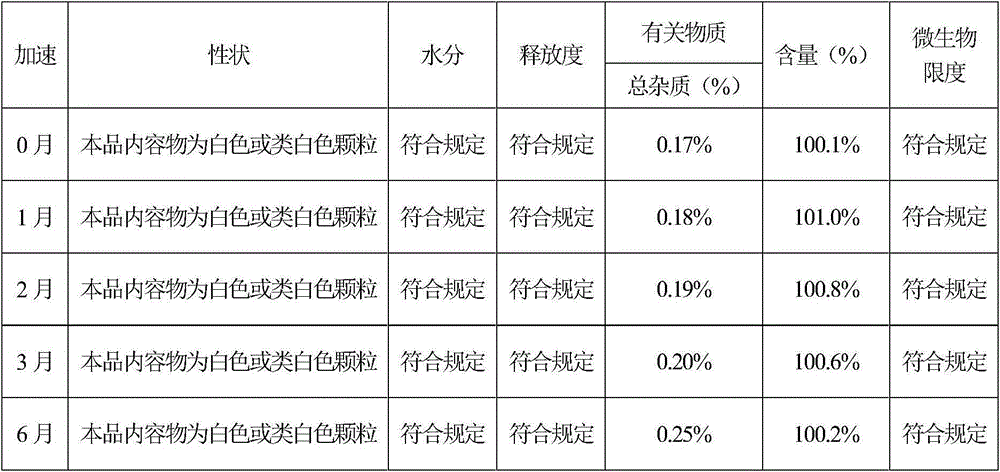
[0071] Preparation process: according to the preparation process of Example 1, it was prepared. According to the test method of Example 1, the release rate measurement and the sample stability test were respectively carried out. The release rate measurement test results showed that levoxiracetam was released slowly, and the release time was as long as 12 hours. The release rate of each sample at different time points RSD All are less than 5%, and the content uniformity RSD of each point is less than 1%. The results of t...
Embodiment 3
[0073] A levooxiracetam sustained-release capsule with good release uniformity is prepared according to the following steps:
[0074] Element Dosage Levooxiracetam 1 serving lactose 1.4 servings Carboxypropyl methylcellulose K4M 1.2 servings Carboxypropyl methylcellulose K15M 1.7 servings carnauba wax 0.6 servings
[0075] octadecanol 0.12 servings 70% ethanol solution 2.5 servings
[0076] 1000 capsules
[0077] Preparation process: according to the preparation process of Example 1, it was prepared. According to the test method of Example 1, the release rate measurement and the sample stability test were respectively carried out. The release rate measurement test results showed that levoxiracetam was released slowly, and the release time was as long as 12 hours. The release rate of each sample at different time points RSD All are less than 5%, and the content uniformity RSD of each point is less than 1...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap