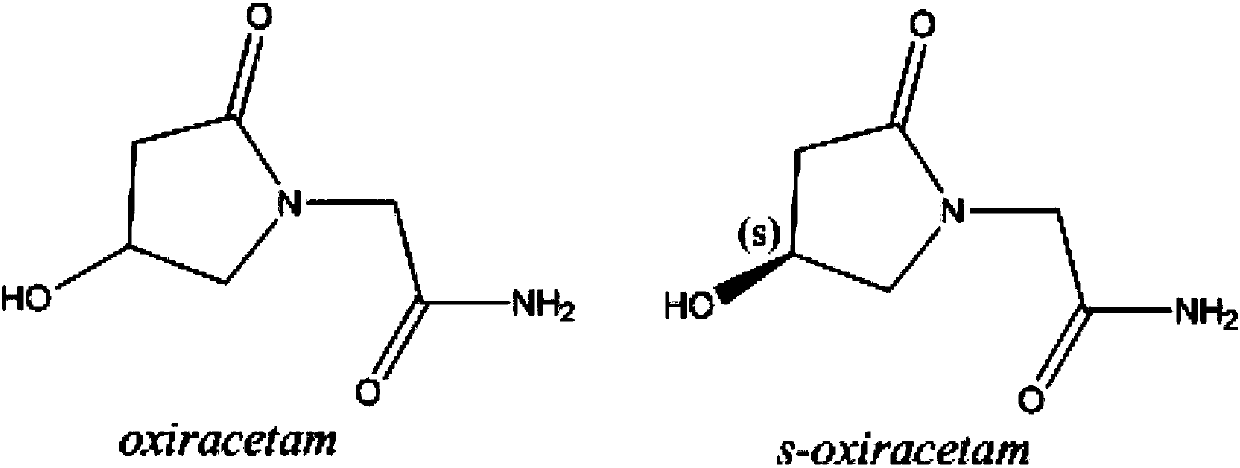
Sustained-release levorotatory oxiracetam capsules and preparation method thereof
A capsule and slow technology, applied in the field of levooxiracetam capsules and preparation thereof, can solve the problems of inability to achieve sustained-release preparations, multiple layers of granules and powders, large differences in release degree, etc., and achieve simple and feasible preparation process and uniform release. Good sex, slow release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
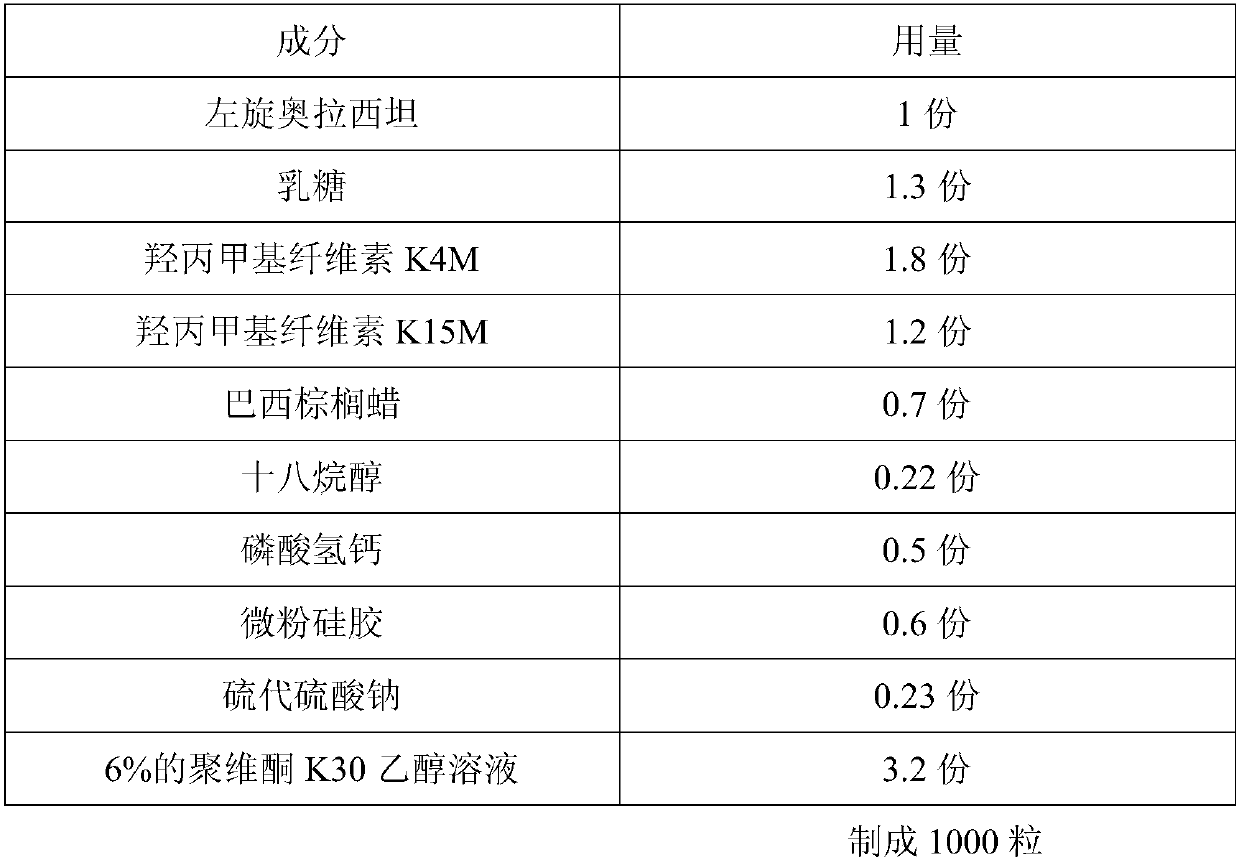
[0023] A slow-release levoxiracetam capsule prepared by the following steps:
[0024]
[0025] Preparation process:
[0026] 1. Pretreatment of raw and auxiliary materials: take the prescribed amount of levoxiracetam, lactose, hydroxypropylmethylcellulose K4M, hydroxypropylmethylcellulose K15M, carnauba wax, calcium hydrogen phosphate, micronized silica gel, sodium thiosulfate Put in the mixing pulverizer and mix and pulverize into fine powder (the amount that can pass through No. 5 sieve and can pass through No. 6 sieve must not be less than 95% of the total amount), and sieve;
[0027] 2. Add povidone K30 ethanol solution, mix and granulate with an 18-mesh sieve, place the wet granules in a hot air oven, set the temperature at 40-60°C, dry until the moisture content of the granules is ≤3%, and granulate (over 24 mesh sieves), standby;
[0028] 3. Total blending: crush stearyl alcohol through a 100-mesh sieve, add it to the granulated granules, and mix with a three-dimen...
Embodiment 2
[0066] A slow-release levoxiracetam capsule prepared by the following steps:
[0067]
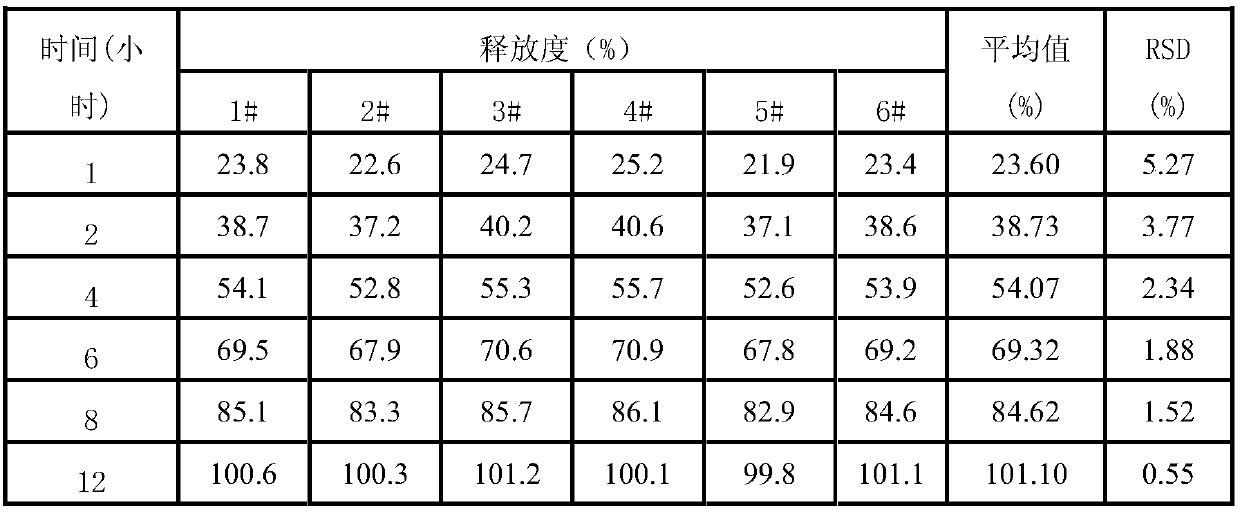
[0068] Preparation process: prepared according to the preparation process of Example 1. Carry out the test by the test method of embodiment 1, the release measurement test result shows that levoxiracetam is slow release, and the release time is up to 12 hours, and the release RSD of each sample at different time points is all less than 5%, and the yield measurement result It shows that the yield of this product is as high as 93.8%, and the yield of this product is good. The stability test results show that the sample quality is stable in June, and the impurity increase is only 0.06%. The quality is stable for 24 months, and the impurity increase is only 0.05%. , There is no capsule adhesion and agglomeration during storage, so the validity period of this product is at least 24 months.
Embodiment 3
[0070] A slow-release levoxiracetam capsule prepared by the following steps:
[0071]
[0072] Preparation process: prepared according to the preparation process of Example 1. Carry out the test by the test method of embodiment 1, the release measurement test result shows that levoxiracetam is slow release, and the release time is up to 12 hours, and the release RSD of each sample at different time points is all less than 6%, and the yield measurement result It shows that the yield of this product is as high as 94.5%, and the yield of this product is good. The stability test results show that the sample quality is stable in June, and the impurity increment is only 0.05%. The long-term 24-month quality is stable, and the impurity increment is only 0.05%. , There is no capsule adhesion and agglomeration during storage, so the validity period of this product is at least 24 months.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap