Sustained release medicinal auxiliary material for improving stability of basic remedy
A technology for pharmaceutical excipients and stability, which is applied in the field of pharmaceutical excipients, can solve the problems such as the lack of L-arginine-α-ketoglutarate and the like, and achieves the effects of good release uniformity and controlled sustained-release behavior.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
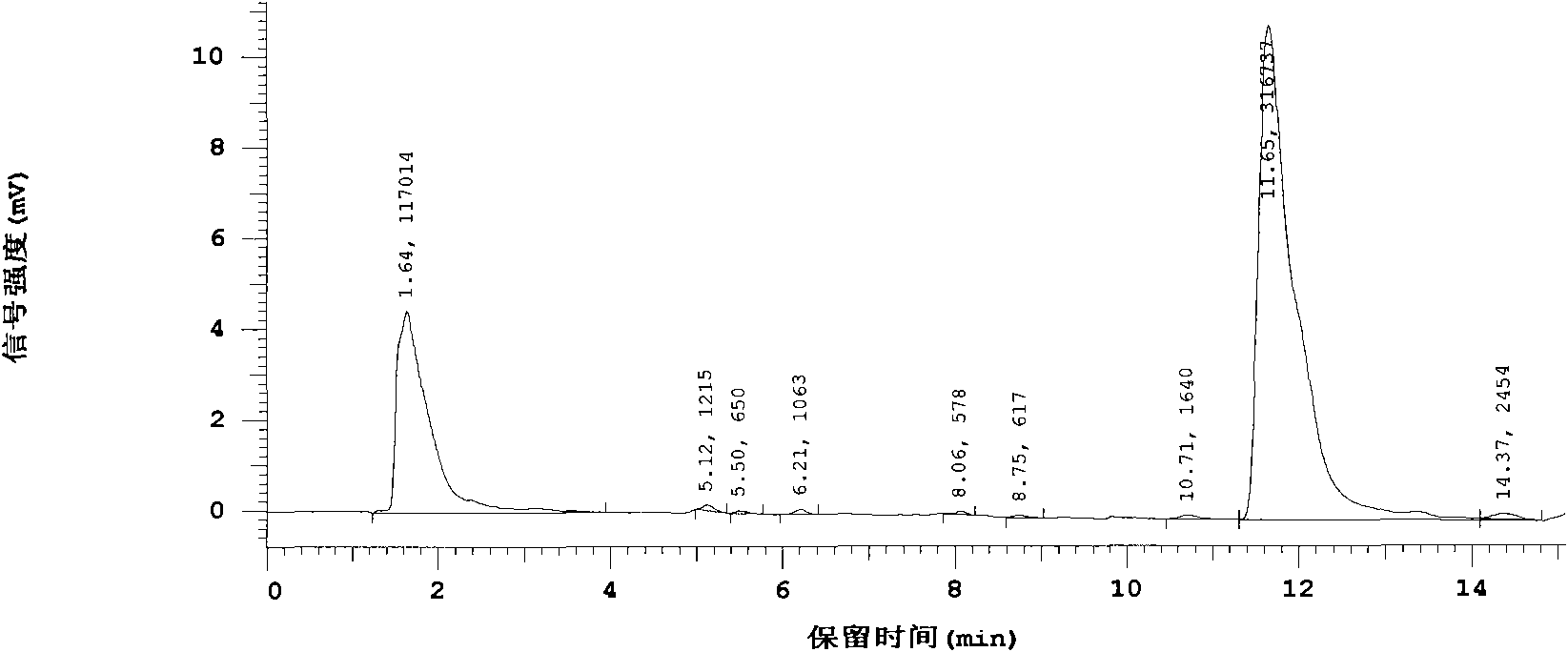
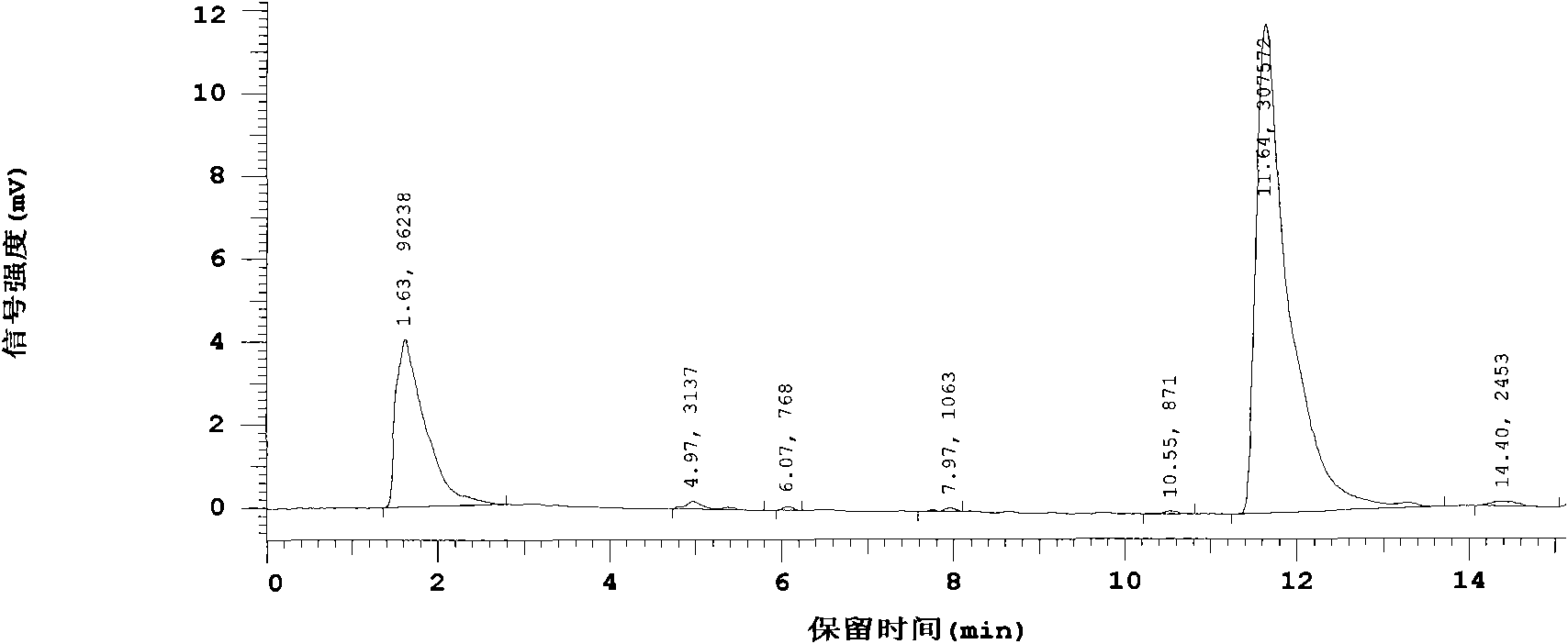
[0020] The crude drug human glucagon-like polypeptide-1 (GLP-1) was made into a sterile aqueous solution, which was equally divided into two parts, one of which was added as a test product with L-arginine-α-ketoglutarate (L -Arginine and α-ketoglutaric acid form a salt with a molar ratio of 2:1), the weight ratio of the added amount to the main drug is 10:1, after the dissolution is complete, carry out aseptic filling as usual, and freeze-dry Finally, it was sealed and made under aseptic conditions; the other was directly aseptically filled as a control product, and the rest of the operations were the same as the test product. After placing it for 12 months, measure its main drug content respectively, and the high-efficiency liquid phase diagram sees figure 2 , image 3 . figure 2 Compared with the liquid chromatogram of the raw material drug, the peak time and peak area are almost the same, and there is only one main peak at 12 minutes, and no other impurity peaks are see...
example 2
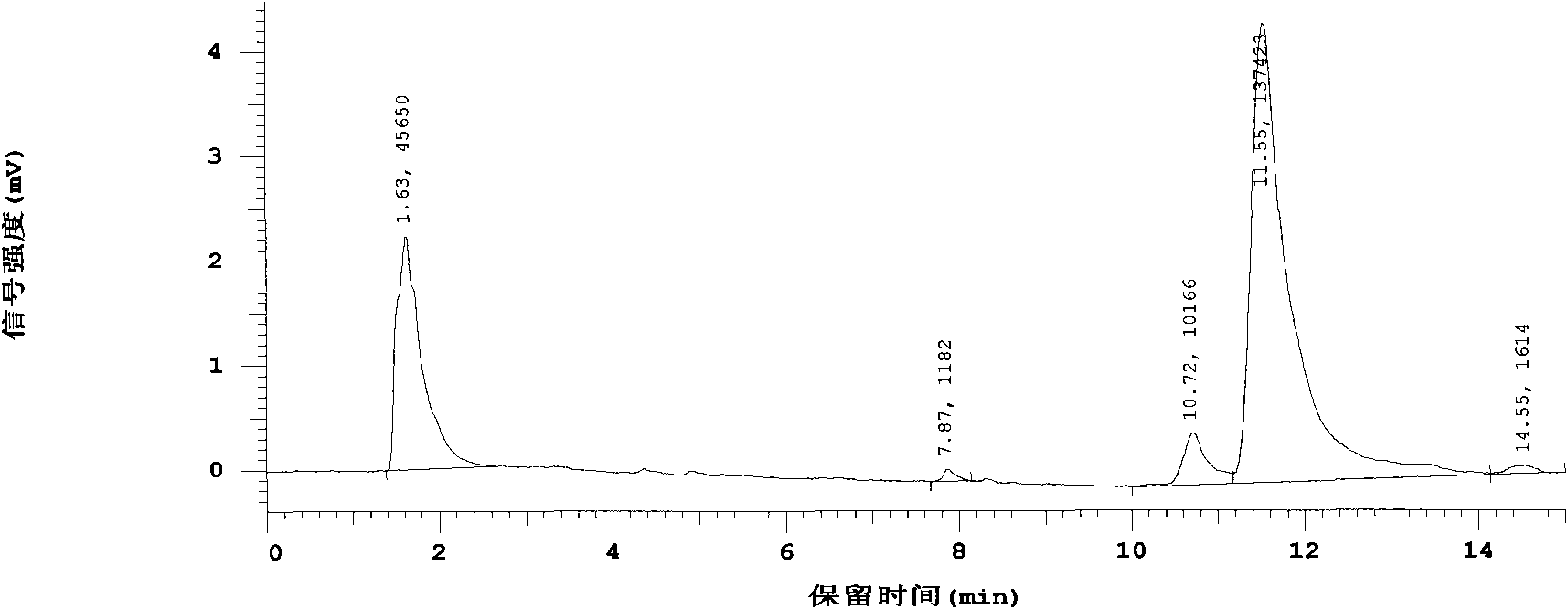
[0022] The crude drug human glucagon-like polypeptide-1 (GLP-1) was made into a sterile aqueous solution, which was equally divided into two parts, one of which was added as a test product with L-arginine-α-ketoglutarate (L -Arginine and α-ketoglutaric acid form a salt with a molar ratio of 2:1), the weight ratio of the added amount to the main drug is 10:1, after the dissolution is complete, carry out aseptic filling as usual, and freeze-dry Finally, it was sealed and made under aseptic conditions; the other was directly aseptically filled as a control product, and the rest of the operations were the same as the test product. Both the test product and the reference substance are dissolved with 5ml of water for injection, first adjust the pH to 10 with sodium hydroxide solution, mix well, then adjust the pH to 4 with hydrochloric acid solution, mix well, take samples respectively to measure the content of the main drug, the results are as follows: Figure 4 , Figure 5 shown....
example 3
[0024] Prepare curcumin sustained-release tablets according to the general tablet preparation process, L-arginine-α-ketoglutarate (L-arginine and α-ketoglutarate form a salt with a molar ratio of 2:1) ) and the weight ratio of raw material curcumin 50: 1, add an appropriate amount of filler starch in addition, disintegrant microcrystalline cellulose, mix uniformly, take polyvinylpyrrolidone K3010% (w / v, 80% ethanol solution) as Wetting agent, granulating, adding appropriate amount of lubricant magnesium stearate after drying, mixing evenly, and compressing into tablets. Refer to Appendix XC of Chinese Pharmacopoeia 2010 Edition, the first method to measure the in vitro drug release characteristics of preparations: 6 samples were randomly taken and placed in 200ml of deionized water degassed by ultrasonic, respectively, and kept at a constant temperature of (37±0.5)°C. The method speed is (100±5)r·min -1 , take 2.0ml of the receiving solution per hour, filter through a micropo...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap